
Given, \[\text{PhC}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}\xrightarrow[\text{(ii)}{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}]{\text{(i)Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{1}}_{\text{2}}}\text{/CC}{{\text{l}}_{\text{4}}}}\text{PhC}{{\text{H}}_{\text{2}}}\text{CHO}\]
The above reaction is an example of which name reaction?
A) Rosenmund reduction
B) Birch reduction
C) Mendius reduction
D) Étard reduction
Answer
590.7k+ views
Hint: The chromyl chloride is an oxidizing agent. It oxidizes the benzylic methyl group by the removal of hydrogen from the alkyl group. The oxygen of chromyl chloride forms a complex called the Étard complex. This complex undergoes the sigmatropic rearrangement to form an aldehyde.
Complete step by step answer:
The reaction which offers a way to oxidize the aromatic methyl’s group $\text{(Ar-C}{{\text{H}}_{\text{3}}}\text{)}$ into an aldehyde $\text{(Ar-CHO)}$ using a chromyl chloride\[\text{Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\]. This oxidation is called the Étard oxidation.
The reaction starts with a reaction of alkene which is formed by the removal of hydrogen and the allylic hydrogen which further reacts with the chromyl chloride to give an Étard complex. This complex is then decomposed by reducing the environment to avoid further oxidation of the aldehyde to a carboxylic acid. Carbon tetrachloride is the most commonly used solvent. Aldehyde obtained by the Étard oxidation is high purity.
The chromyl chloride forms an Étard complex.
An end reaction with chromyl chloride forming a precipitated Étard Complex is the first step of this mechanism.
The sigmatropic reaction is a pericyclic reaction where one$\text{ }\!\!\sigma\!\!\text{ }$ bond is converted into the other $\text{ }\!\!\sigma\!\!\text{ }$ bond. The methyl is converted into the aldehyde through the $\left[ 2,3 \right]$ sigmatropic rearrangement reaction.
The Étard complex formed is decomposed into the Phenyl acetaldehyde.
This is a very easy method for the oxidation of ethylbenzene into the Phenyl acetaldehyde.
Thus the given reaction,
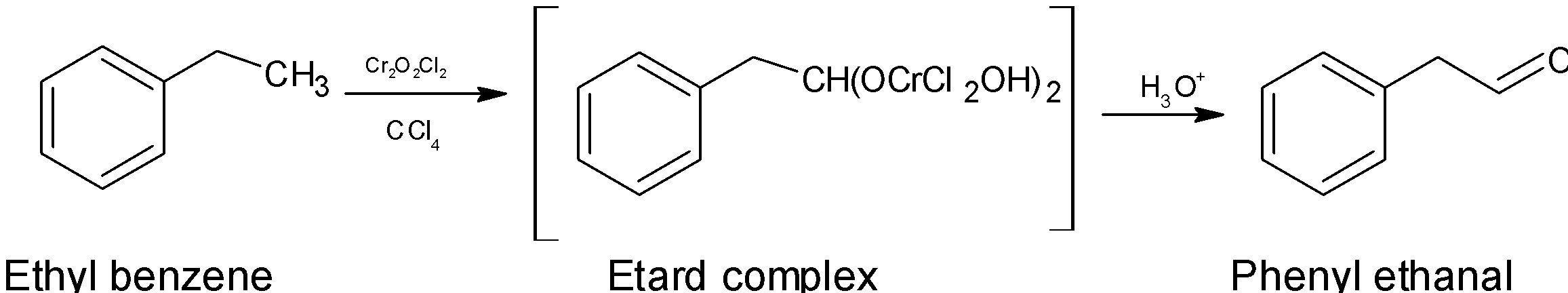
The conversion of ethylbenzene to Phenyl acetaldehyde is Étard oxidation.
So, the correct answer is “Option D”.
Note: The Étard complex can undergo the rapid oxidation to give carboxylic acid, therefore used as a reducing agent to decompose complex.
Sigmatropic rearrangement makes it difficult for the other alkyl benzene to formaldehyde.
Complete step by step answer:
The reaction which offers a way to oxidize the aromatic methyl’s group $\text{(Ar-C}{{\text{H}}_{\text{3}}}\text{)}$ into an aldehyde $\text{(Ar-CHO)}$ using a chromyl chloride\[\text{Cr}{{\text{O}}_{\text{2}}}\text{C}{{\text{l}}_{\text{2}}}\]. This oxidation is called the Étard oxidation.
The reaction starts with a reaction of alkene which is formed by the removal of hydrogen and the allylic hydrogen which further reacts with the chromyl chloride to give an Étard complex. This complex is then decomposed by reducing the environment to avoid further oxidation of the aldehyde to a carboxylic acid. Carbon tetrachloride is the most commonly used solvent. Aldehyde obtained by the Étard oxidation is high purity.
The chromyl chloride forms an Étard complex.
An end reaction with chromyl chloride forming a precipitated Étard Complex is the first step of this mechanism.
The sigmatropic reaction is a pericyclic reaction where one$\text{ }\!\!\sigma\!\!\text{ }$ bond is converted into the other $\text{ }\!\!\sigma\!\!\text{ }$ bond. The methyl is converted into the aldehyde through the $\left[ 2,3 \right]$ sigmatropic rearrangement reaction.
The Étard complex formed is decomposed into the Phenyl acetaldehyde.
This is a very easy method for the oxidation of ethylbenzene into the Phenyl acetaldehyde.
Thus the given reaction,

The conversion of ethylbenzene to Phenyl acetaldehyde is Étard oxidation.
So, the correct answer is “Option D”.
Note: The Étard complex can undergo the rapid oxidation to give carboxylic acid, therefore used as a reducing agent to decompose complex.
Sigmatropic rearrangement makes it difficult for the other alkyl benzene to formaldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




