
Give the relative stability of the following compound
i

ii
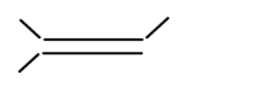
iii
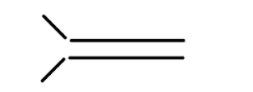
iv
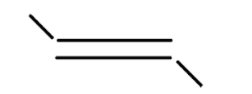
v
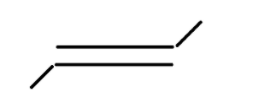
vi

vii








Answer
550.8k+ views
Hint: Relative stability in organic compounds means the ability of the compound to resist itself from changing or altering to other compounds. The compounds in chemistry usually change themselves to their stable energy state. If the structure has least separation of formal charge then the compound is usually said to be stable.
Complete step by step solution:
In alkene the stability of the compound is determined by the number of alpha hydrogen atoms present in the compound. Increase in the number of alpha hydrogen atoms will increase the hyperconjugation of the compound thereby increasing the stability of the compound. So in part (i) the number of alpha hydrogen atoms is 12. In part(ii) the number of alpha hydrogen atoms is 9. In part (iii) the number of alpha hydrogen atoms is 6. In part (iv) the number alpha hydrogen atom is 6. In part (v) the number of alpha hydrogen atoms is 6. In part (vi) the number of alpha hydrogen atoms is 3. In part (vii) the number of alpha hydrogen atoms is 0. So according to this the highest alpha hydrogen atom is in the first part that is 12. So this compound will be relatively most stable and (vii) will be least stable as it has no alpha hydrogen atom.
Trans alkenes are more stable than cis alkenes because they have less steric interactions. Due to this trans isomers have less exothermic heat of combustion in comparison to cis alkenes. So the relative stability of compounds is
So, the correct answer is i>ii>iv>v>iii>vi>vii
Note: The hyperconjugation is the effect which is a temporary effect done in the presence of attacking reagent. It is done at the vicinity of the organic compound with multiple bonds. In this the transfer of the shared pair of $$\Pi electron$$ takes place. The effect ceases as soon as the attacking reagent is taken out.
Complete step by step solution:
In alkene the stability of the compound is determined by the number of alpha hydrogen atoms present in the compound. Increase in the number of alpha hydrogen atoms will increase the hyperconjugation of the compound thereby increasing the stability of the compound. So in part (i) the number of alpha hydrogen atoms is 12. In part(ii) the number of alpha hydrogen atoms is 9. In part (iii) the number of alpha hydrogen atoms is 6. In part (iv) the number alpha hydrogen atom is 6. In part (v) the number of alpha hydrogen atoms is 6. In part (vi) the number of alpha hydrogen atoms is 3. In part (vii) the number of alpha hydrogen atoms is 0. So according to this the highest alpha hydrogen atom is in the first part that is 12. So this compound will be relatively most stable and (vii) will be least stable as it has no alpha hydrogen atom.
Trans alkenes are more stable than cis alkenes because they have less steric interactions. Due to this trans isomers have less exothermic heat of combustion in comparison to cis alkenes. So the relative stability of compounds is
So, the correct answer is i>ii>iv>v>iii>vi>vii
Note: The hyperconjugation is the effect which is a temporary effect done in the presence of attacking reagent. It is done at the vicinity of the organic compound with multiple bonds. In this the transfer of the shared pair of $$\Pi electron$$ takes place. The effect ceases as soon as the attacking reagent is taken out.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




