
Give the correct order of increasing acidity of the following compounds.
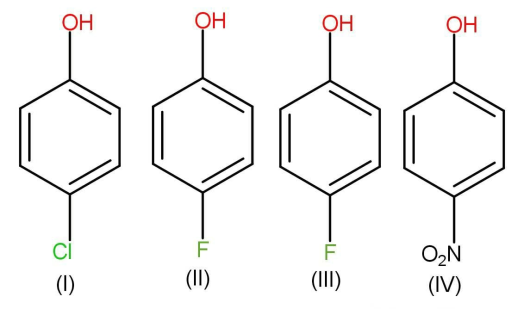
A.\[{\text{III}} < {\text{I}} < {\text{II}} < {\text{IV}}\]
B.\[{\text{III}} < {\text{II}} < {\text{I}} < {\text{IV}}\]
C.\[{\text{III}} < {\text{I}} < {\text{IV}} < {\text{II}}\]
D.\[{\text{IV}} < {\text{II}} < {\text{I}} < {\text{III}}\]

Answer
574.8k+ views
Hint: Electron donating group tends to decrease the acidic strength of phenols and electron withdrawing group tends to increase the acidic strength phenols. The more electron donating tendency any group has, the less acidic it will be with less acidic strength.
Complete step by step answer:
An acid is formed by the removal of hydrogen ions from a molecule. Now if the hydrogen ions are removed from phenol and phenoxide ion is formed which is an anion. The stability of this anion will determine whether the compound will be more acidic or less acidic. The more stable this negative charge will be, more will be the acidic character. Electron donating groups tend to increase the electron density hence destabilising the negative charge and makes the molecule less acidic whereas the electron withdrawing makes the compound more acidic by withdrawing some negative charge from the molecule and stabilises it.
Methyl donates electrons and shows inductive effect whereas chloride, fluoride and nitro groups are electron withdrawing groups. The third molecule will be least acidic because of the presence of an electron donating group that is methyl.
Among chloride, fluoride and nitro groups, nitro groups withdraw electrons through resonance whereas in case of halogen or halide minus I effect dominates. That is the chloride and fluoride will take electrons or withdraw electrons for inductive effect. The more is the electronegativity of the element the more will be the inductive effect. Inductive effect is always less effective in comparison to resonance. Hence the molecule 4 will be the most stable because it will take electrons to a maximum extent due to resonance. Now among chloride and fluoride chlorine has less electronegativity than fluorine. But still the chlorophenol is more acidic. This is due to the small size of fluorine and presence of vacant d orbital in chlorine.
Hence, the correct order is:
\[{\text{III}} < {\text{II}} < {\text{I}} < {\text{IV}}\], that is, option B.
Note:
If any functional group tends to donate electrons or withdraw electrons by resonance such effect is known as mesomeric effect. The group which donates the electrons to the carbon chain through resonance are known as \[ + {\text{M}}\] groups and the group or atoms which withdraw electron from carbon chain or ring are known as \[ - {\text{M}}\] effect groups.
Complete step by step answer:
An acid is formed by the removal of hydrogen ions from a molecule. Now if the hydrogen ions are removed from phenol and phenoxide ion is formed which is an anion. The stability of this anion will determine whether the compound will be more acidic or less acidic. The more stable this negative charge will be, more will be the acidic character. Electron donating groups tend to increase the electron density hence destabilising the negative charge and makes the molecule less acidic whereas the electron withdrawing makes the compound more acidic by withdrawing some negative charge from the molecule and stabilises it.
Methyl donates electrons and shows inductive effect whereas chloride, fluoride and nitro groups are electron withdrawing groups. The third molecule will be least acidic because of the presence of an electron donating group that is methyl.
Among chloride, fluoride and nitro groups, nitro groups withdraw electrons through resonance whereas in case of halogen or halide minus I effect dominates. That is the chloride and fluoride will take electrons or withdraw electrons for inductive effect. The more is the electronegativity of the element the more will be the inductive effect. Inductive effect is always less effective in comparison to resonance. Hence the molecule 4 will be the most stable because it will take electrons to a maximum extent due to resonance. Now among chloride and fluoride chlorine has less electronegativity than fluorine. But still the chlorophenol is more acidic. This is due to the small size of fluorine and presence of vacant d orbital in chlorine.
Hence, the correct order is:
\[{\text{III}} < {\text{II}} < {\text{I}} < {\text{IV}}\], that is, option B.
Note:
If any functional group tends to donate electrons or withdraw electrons by resonance such effect is known as mesomeric effect. The group which donates the electrons to the carbon chain through resonance are known as \[ + {\text{M}}\] groups and the group or atoms which withdraw electron from carbon chain or ring are known as \[ - {\text{M}}\] effect groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




