
Give simple chemical test to distinguish between the following pair of compounds
i.Ethanal and propanal
ii.Benzoic acid and phenol
Answer
520.8k+ views
Hint: We have to remember that the ethanal is an organic compound and it is also known as acetaldehyde having the formula, \[C{H_3}CHO\]. And the propanal is also an aldehyde which is known as propionaldehyde having the formula, \[C{H_3}C{H_2}CHO\]. Benzoic acid is an organic compound with the chemical formula, \[{C_6}{H_5}COOH\]. And phenol is an aromatic organic compound having the formula, \[{C_6}{H_5}OH\].
Complete answer:
(i) Ethanal and propanal can be distinguished by iodoform test. The iodoform test is used to find out the presence of carbonyl compounds. And ethanal is only the aldehyde which gives the iodoform test and there is a formation of yellow precipitate. When the ethanal is reacting with the iodoform mixture, that is a mixture of sodium hydroxide and iodine and there is a formation of methyl iodide which gives the yellow precipitate. The reaction can be written as,
\[C{H_3}CHO + 4NaOH + 3{I_2} \to 3C{H_3}I + HCOONa + 3NaI + 3{H_2}O\]
But, propanal will not give the iodoform test and it will not give any yellow precipitate.
\[C{H_3}C{H_2}CHO + 4NaOH + 3{I_2} \to No\,reaction\]
(ii) Benzoic acid and phenol can be distinguished by sodium bicarbonate test. Benzoic acid gives sodium bicarbonate test. When benzoic acid reacts in sodium bicarbonate, there is a formation of brisk effervescence by evolving \[C{O_2}\] gas. And the reaction can be written as,
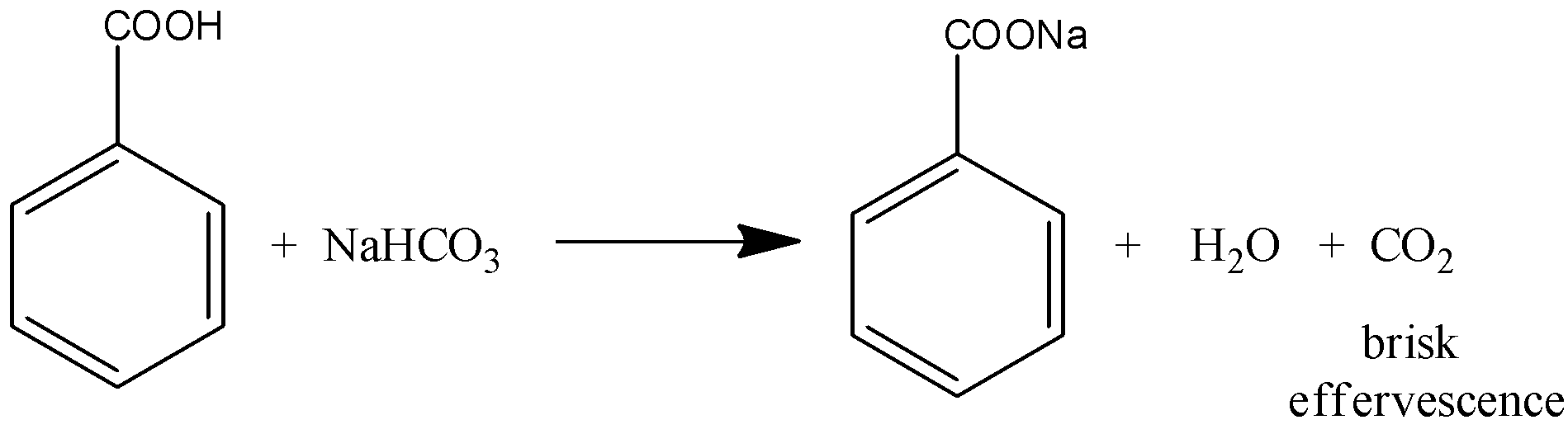
But phenol does not give a positive result for sodium bicarbonate test.
Note:
We have to remember that the ethanal and propanal can be distinguished by iodoform test. Propanal will not give the iodoform test and it will not give any yellow precipitate but ethanal gives a yellow precipitate which is methyl iodide, \[C{H_3}I\]. And benzoic acid and phenol can be distinguished by using sodium bicarbonate tests. Benzoic acid gives brisk effervescence by evolving \[C{O_2}\] gas but Phenol does not give a positive result for sodium bicarbonate test.
Complete answer:
(i) Ethanal and propanal can be distinguished by iodoform test. The iodoform test is used to find out the presence of carbonyl compounds. And ethanal is only the aldehyde which gives the iodoform test and there is a formation of yellow precipitate. When the ethanal is reacting with the iodoform mixture, that is a mixture of sodium hydroxide and iodine and there is a formation of methyl iodide which gives the yellow precipitate. The reaction can be written as,
\[C{H_3}CHO + 4NaOH + 3{I_2} \to 3C{H_3}I + HCOONa + 3NaI + 3{H_2}O\]
But, propanal will not give the iodoform test and it will not give any yellow precipitate.
\[C{H_3}C{H_2}CHO + 4NaOH + 3{I_2} \to No\,reaction\]
(ii) Benzoic acid and phenol can be distinguished by sodium bicarbonate test. Benzoic acid gives sodium bicarbonate test. When benzoic acid reacts in sodium bicarbonate, there is a formation of brisk effervescence by evolving \[C{O_2}\] gas. And the reaction can be written as,

But phenol does not give a positive result for sodium bicarbonate test.
Note:
We have to remember that the ethanal and propanal can be distinguished by iodoform test. Propanal will not give the iodoform test and it will not give any yellow precipitate but ethanal gives a yellow precipitate which is methyl iodide, \[C{H_3}I\]. And benzoic acid and phenol can be distinguished by using sodium bicarbonate tests. Benzoic acid gives brisk effervescence by evolving \[C{O_2}\] gas but Phenol does not give a positive result for sodium bicarbonate test.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




