
Give reasons:
“The presence of nitro group $\left( { - {\rm{N}}{{\rm{O}}_2}} \right)$ at ortho/para positions increase the reactivity of haloarenes towards nucleophilic substitution reaction”.
Answer
561k+ views
Hint: We know that nucleophilic substitution reaction is the reaction in which a nucleophile attacks an electrophile (positively charged) to replace a leaving group. A nucleophile is an electron rich species and an electrophile is an electron deficient species.
Complete step by step answer:
We know that the nitro group is an electron withdrawing group. When a nitro group is present at ortho and para position, it can withdraw electrons from the benzene ring. This facilitates the attack of nucleophiles on haloarene. The carbanion formed due to the attack of the nucleophile got stabilized by resonance. The negative charge appeared at the ortho and para position with respect to the halogen atom is stabilized by the nitro group.
Let’s understand with the help of a diagram.
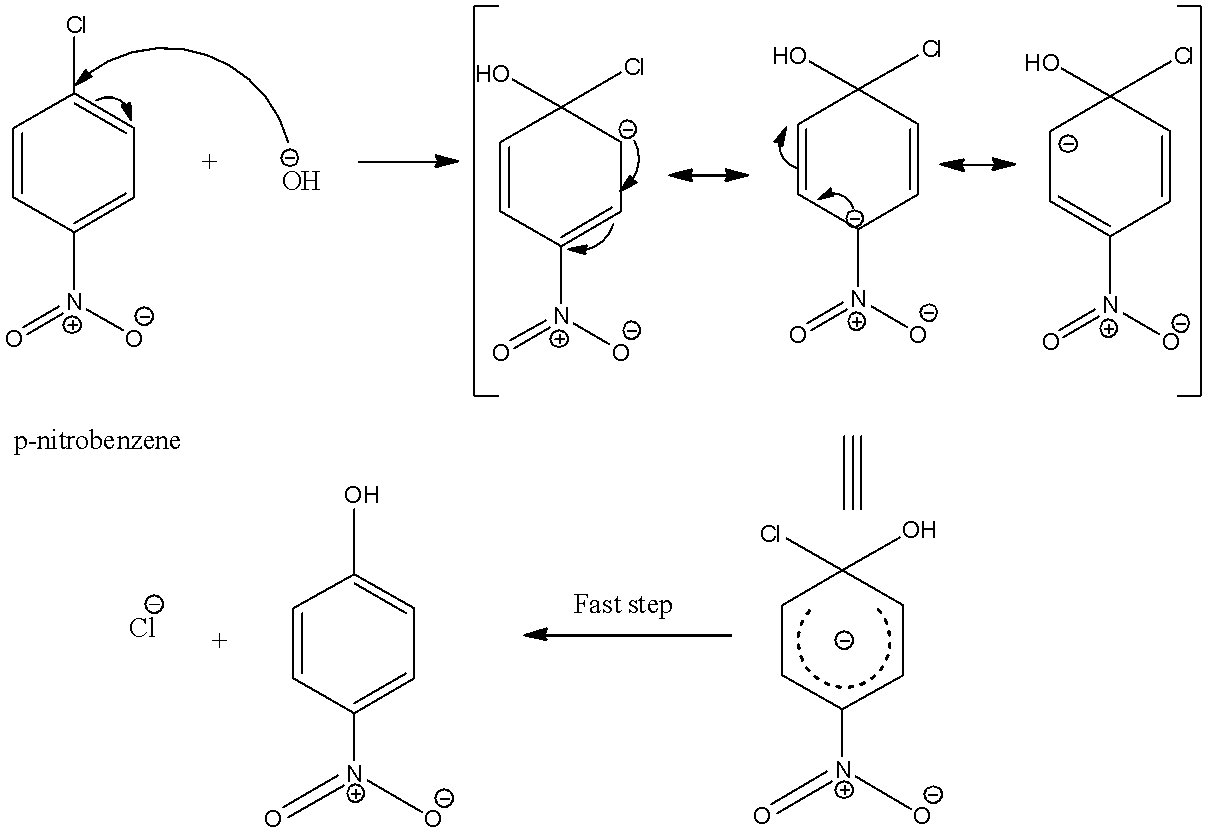
Here, one of the resonating structures possess a negative charge on the carbon atom bonded to the nitro group. So, the negative charge stabilized.
Similarly in case of ortho-nitrobenzene,
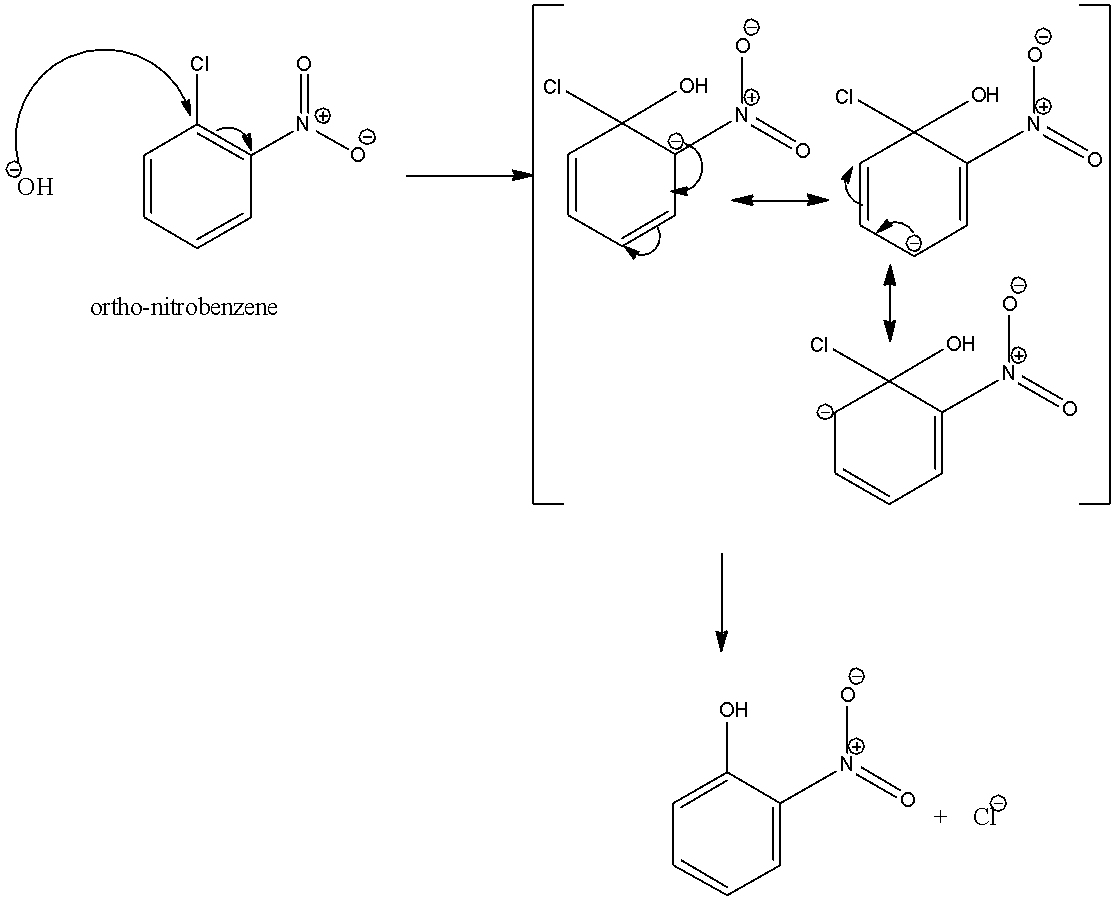
Here, one of the resonating structures possesses negative charge on the carbon atom bonded to the nitro group. So, the negative charge is stabilized. And hence, the reactivity of haloarenes towards the nucleophilic substitution increases if the nitro group is present at ortho and meta position.
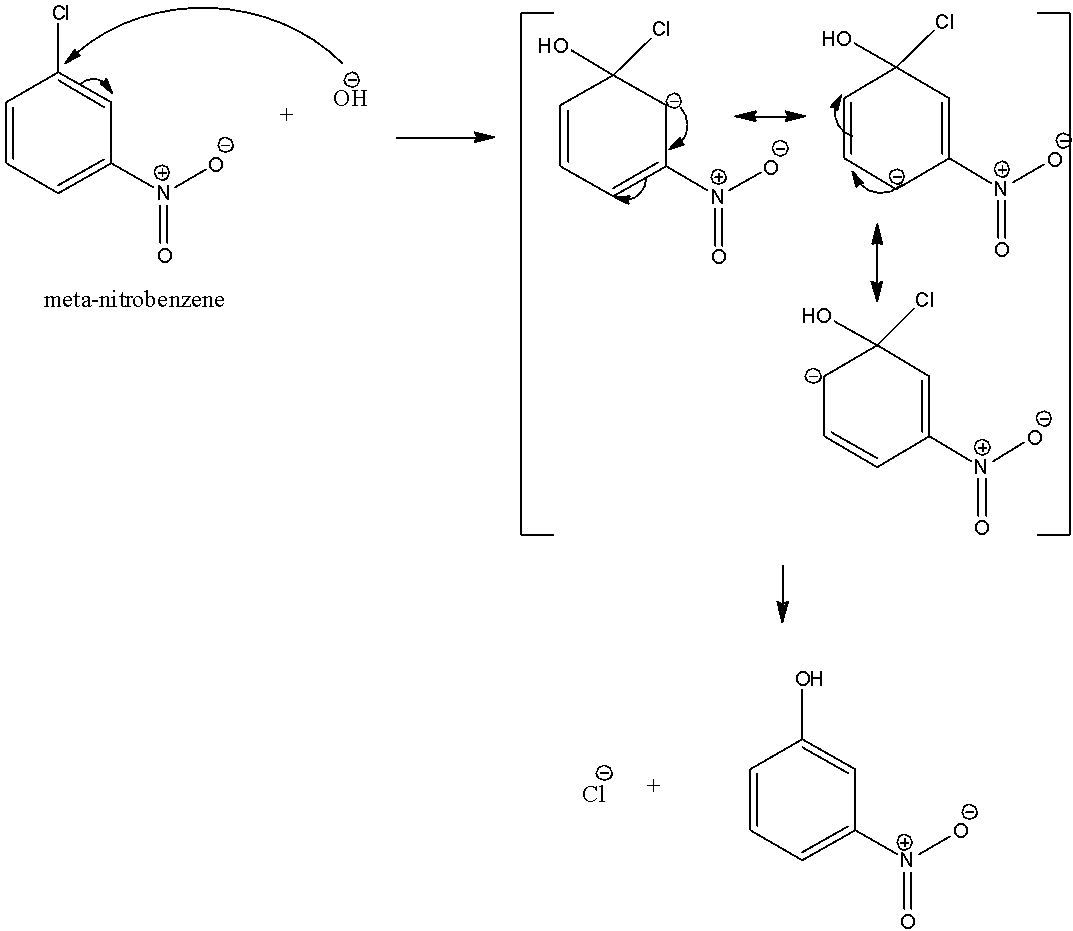
Note: In case meta-nitrobenzene, none of the resonating structures possesses the negative charge on the carbon atom which is bonded to the nitro group. Therefore, nitro group at meta position does not stabilize the negative charge and therefore no effect of nitro group on reactivity is found in case of meta nitrobenzene.
Complete step by step answer:
We know that the nitro group is an electron withdrawing group. When a nitro group is present at ortho and para position, it can withdraw electrons from the benzene ring. This facilitates the attack of nucleophiles on haloarene. The carbanion formed due to the attack of the nucleophile got stabilized by resonance. The negative charge appeared at the ortho and para position with respect to the halogen atom is stabilized by the nitro group.
Let’s understand with the help of a diagram.

Here, one of the resonating structures possess a negative charge on the carbon atom bonded to the nitro group. So, the negative charge stabilized.
Similarly in case of ortho-nitrobenzene,

Here, one of the resonating structures possesses negative charge on the carbon atom bonded to the nitro group. So, the negative charge is stabilized. And hence, the reactivity of haloarenes towards the nucleophilic substitution increases if the nitro group is present at ortho and meta position.
Note: In case meta-nitrobenzene, none of the resonating structures possesses the negative charge on the carbon atom which is bonded to the nitro group. Therefore, nitro group at meta position does not stabilize the negative charge and therefore no effect of nitro group on reactivity is found in case of meta nitrobenzene.

Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




