
Give reasons.
Iron has higher enthalpy of atomization than that of copper.
Answer
592.2k+ views
Hint: Enthalpy of atomization directly depends upon the strength of the metallic bond which is further dependent on the number of the unpaired electrons present in the outermost shell of the element. Hence, greater the number of unpaired electrons present, stronger will be the metallic bonding and therefore higher will be the enthalpy of atomization.
Complete answer: Enthalpy of atomization directly depends upon the strength of the metallic bond which is further dependent on the number of the unpaired electrons present in the outermost shell of the element. Hence, greater the number of unpaired electrons present, stronger will be the metallic bonding and therefore higher will be the enthalpy of atomization.
Iron and copper are transition elements and belong to the d block. The atomic numbers of iron and copper are 26 and 29, simultaneously. The iron element belongs to the Group 8 and copper belongs to the Group 11 of the periodic table. According to the Aufbau principle, the electronic configuration of iron is $[Ar]3{{d}^{6}}4{{s}^{2}}$ and the electronic configuration of copper must be $\left[ Ar \right]3{{d}^{9}}4{{s}^{2}}$ but based on the fact that fully filled orbitals exhibit higher stability so the electronic configuration of copper element is $\left[ Ar \right]3{{d}^{10}}4{{s}^{1}}$.
Pictorial representation of filling of electrons:
Electronic configuration of iron
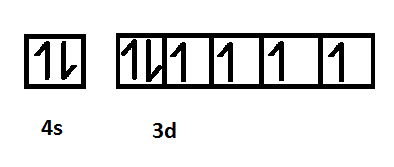
Electronic configuration of copper
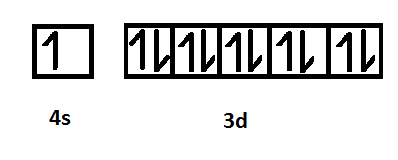
The enthalpy of atomization is the change in enthalpy that occurs when all the bonds in a compound break to form individual atoms. The enthalpy of atomization is directly related to the strength of the metallic bonds which is further associated with the number of unpaired electrons present in the outermost shell of the element. Therefore, the enthalpy of atomization depends on the number of unpaired electrons present in the valence shell. As illustrated in the pictorial representation, the iron metal consists of 4 unpaired electrons and copper metal consists of 1 unpaired electron. Therefore, iron consists of a greater number of electrons as compared to copper due to which iron exhibits stronger metallic bonding and as a result, will have higher enthalpy of atomization than that of copper.
Additional Information: The enthalpy of atomization of iron is 347 kJ$mo{{l}^{-1}}$. The enthalpy of atomization of copper is 338 kJ$mo{{l}^{-1}}$.
Note: The element chromium with atomic number 24 also shows exceptional electronic configuration similar to the copper. The electronic configuration of chromium should be $\left[ Ar \right]3{{d}^{4}}4{{s}^{2}}$ but due to the stability attained by symmetrical distribution of electrons (half-filled d-orbitals) and by exchange energy, the electronic configuration of chromium is \[\left[ Ar \right]3{{d}^{5}}4{{s}^{1}}\].
Complete answer: Enthalpy of atomization directly depends upon the strength of the metallic bond which is further dependent on the number of the unpaired electrons present in the outermost shell of the element. Hence, greater the number of unpaired electrons present, stronger will be the metallic bonding and therefore higher will be the enthalpy of atomization.
Iron and copper are transition elements and belong to the d block. The atomic numbers of iron and copper are 26 and 29, simultaneously. The iron element belongs to the Group 8 and copper belongs to the Group 11 of the periodic table. According to the Aufbau principle, the electronic configuration of iron is $[Ar]3{{d}^{6}}4{{s}^{2}}$ and the electronic configuration of copper must be $\left[ Ar \right]3{{d}^{9}}4{{s}^{2}}$ but based on the fact that fully filled orbitals exhibit higher stability so the electronic configuration of copper element is $\left[ Ar \right]3{{d}^{10}}4{{s}^{1}}$.
Pictorial representation of filling of electrons:
Electronic configuration of iron

Electronic configuration of copper

The enthalpy of atomization is the change in enthalpy that occurs when all the bonds in a compound break to form individual atoms. The enthalpy of atomization is directly related to the strength of the metallic bonds which is further associated with the number of unpaired electrons present in the outermost shell of the element. Therefore, the enthalpy of atomization depends on the number of unpaired electrons present in the valence shell. As illustrated in the pictorial representation, the iron metal consists of 4 unpaired electrons and copper metal consists of 1 unpaired electron. Therefore, iron consists of a greater number of electrons as compared to copper due to which iron exhibits stronger metallic bonding and as a result, will have higher enthalpy of atomization than that of copper.
Additional Information: The enthalpy of atomization of iron is 347 kJ$mo{{l}^{-1}}$. The enthalpy of atomization of copper is 338 kJ$mo{{l}^{-1}}$.
Note: The element chromium with atomic number 24 also shows exceptional electronic configuration similar to the copper. The electronic configuration of chromium should be $\left[ Ar \right]3{{d}^{4}}4{{s}^{2}}$ but due to the stability attained by symmetrical distribution of electrons (half-filled d-orbitals) and by exchange energy, the electronic configuration of chromium is \[\left[ Ar \right]3{{d}^{5}}4{{s}^{1}}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




