
Give condensed and bond line structural formula and identify functional group (s) present, if any, for Hexanedial.
Answer
573.6k+ views
Hint: First of all look at the name of the compound then you will come to know from the suffix and prefix that exactly what the compound is. And what are the functional groups attached to it and in which manner are they joined or combined.
Complete Step by step answer: First of all we should know what the meanings of the following terms are,
So Condensed structural formulas are those formulas which show the order of atoms like a structural formula but are written in a single line to save space and make it more convenient and faster to write out. Condensed structural formulas are also helpful when showing that a group of atoms is connected to a single atom in a compound.
And Bond-line structure (bond-line formula, skeletal structure, and skeletal formula) is a representation of molecular structure in which covalent bonds are represented with one line for each level of bond order. A single bond is represented with a single line, a double bond is shown by two parallel lines, and a triple bond by three parallel lines.
In organic chemistry, functional groups are specific substituents within molecules that may be responsible for the characteristic chemical reaction of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of.
So according to the above information Hexanedial will have,
Condensed Formula: $(OHC){(C{H_2})_4}(CHO)$
Bond line structural formula:
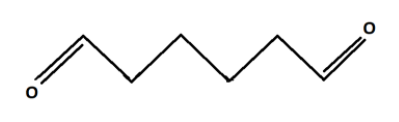
Functional Group: Two Aldehyde Groups
Note: Functional groups in organic compounds are responsible for their chemical properties. If the compound is having the same functional group but the carbon chain attached with it is different, even in this case that compound will show similar properties.
Complete Step by step answer: First of all we should know what the meanings of the following terms are,
So Condensed structural formulas are those formulas which show the order of atoms like a structural formula but are written in a single line to save space and make it more convenient and faster to write out. Condensed structural formulas are also helpful when showing that a group of atoms is connected to a single atom in a compound.
And Bond-line structure (bond-line formula, skeletal structure, and skeletal formula) is a representation of molecular structure in which covalent bonds are represented with one line for each level of bond order. A single bond is represented with a single line, a double bond is shown by two parallel lines, and a triple bond by three parallel lines.
In organic chemistry, functional groups are specific substituents within molecules that may be responsible for the characteristic chemical reaction of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of.
So according to the above information Hexanedial will have,
Condensed Formula: $(OHC){(C{H_2})_4}(CHO)$
Bond line structural formula:

Functional Group: Two Aldehyde Groups
Note: Functional groups in organic compounds are responsible for their chemical properties. If the compound is having the same functional group but the carbon chain attached with it is different, even in this case that compound will show similar properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




