
Give chemical test to distinguish: (i) Ethyl alcohol and sec-propyl alcohol (ii) Acetaldehyde and acetic acid
Answer
572.1k+ views
Hint: (i) We can select a reagent that reacts at different rates with primary and secondary alcohols. That test will provide the required difference.
(ii)We have to select a reagent that precipitates with one compound but does not react with another compound.
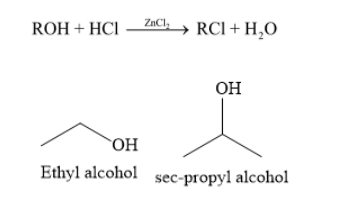
Complete Step by step answer: (i) Ethyl alcohol is a primary alcohol and sec-propyl alcohol is a secondary alcohol. To distinguish ethyl alcohol and sec-propyl alcohol, you can carry out Lucas test. Lucas test is used to distinguish between primary, secondary and tertiary alcohols. Lucas reagent is a mixture of concentric hydrochloric acid and anhydrous zinc chloride. At room temperature ethyl alcohol (a primary alcohol) does not form any turbidity with Lucas reagent.
Sec-propyl alcohol (a secondary alcohol) forms any turbidity with Lucas reagent within 5-10 minutes.
Lucas reagent converts an alcohol to alkyl halide. The turbidity formed during Lucas test is due to formation of alkyl halide.
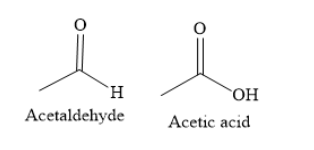
(ii) To distinguish acetaldehyde and acetic acid you can use a silver mirror test.
You can use Tollen’s reagent to carry out a silver mirror test. The Tollen’s reagent is an ammoniacal silver nitrate solution. In the presence of Tollen’s reagent, acetaldehyde is oxidized to acetate ion. Silver nitrate is reduced to silver metal. A black ring of silver is formed in the test tube. This black ring is called a silver mirror.
Acetic acid does not react with Tollen’s reagent.
Note: It is possible to distinguish two compounds by using appropriate reagent. This is possible because two compounds contain different functional groups that react differently with appropriate reagent.
(ii)We have to select a reagent that precipitates with one compound but does not react with another compound.
Complete Step by step answer: (i) Ethyl alcohol is a primary alcohol and sec-propyl alcohol is a secondary alcohol. To distinguish ethyl alcohol and sec-propyl alcohol, you can carry out Lucas test. Lucas test is used to distinguish between primary, secondary and tertiary alcohols. Lucas reagent is a mixture of concentric hydrochloric acid and anhydrous zinc chloride. At room temperature ethyl alcohol (a primary alcohol) does not form any turbidity with Lucas reagent.
Sec-propyl alcohol (a secondary alcohol) forms any turbidity with Lucas reagent within 5-10 minutes.
Lucas reagent converts an alcohol to alkyl halide. The turbidity formed during Lucas test is due to formation of alkyl halide.

(ii) To distinguish acetaldehyde and acetic acid you can use a silver mirror test.
You can use Tollen’s reagent to carry out a silver mirror test. The Tollen’s reagent is an ammoniacal silver nitrate solution. In the presence of Tollen’s reagent, acetaldehyde is oxidized to acetate ion. Silver nitrate is reduced to silver metal. A black ring of silver is formed in the test tube. This black ring is called a silver mirror.
Acetic acid does not react with Tollen’s reagent.

Note: It is possible to distinguish two compounds by using appropriate reagent. This is possible because two compounds contain different functional groups that react differently with appropriate reagent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




