
Formula of the bleaching powder is:
A. \[Ca{{(OH)}_{2}}\]
B. \[CHC{{l}_{3}}\]
C. \[CC{{l}_{3}}CHO\]
D. \[CaOC{{l}_{2}}\]
Answer
594k+ views
Hint: Bleaching powder contains two chlorine atoms. Out of two chlorine atoms one is bonded to the calcium atoms and the other bonded to the oxygen atom. The molecular mass of bleaching powder is 142.98 g/mol.
Bleaching powder is called chloride of lime or chlorinated lime.
Complete step by step answer:
(I)Calcium hypochlorite is the chemical name of bleaching powder.
(II)The chemical formula of bleaching powder is \[CaOC{{l}_{2}}\].
(III)The structure of bleaching powder is as follows.
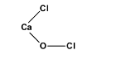
Hence, the correct answer is option D.
Additional Information
(i)Bleaching powder is pale yellow in color and exhibits a strong odor of chlorine.
(ii)Bleaching powder is soluble in water but due to the existence of impurities, we cannot observe a clear solution.
(iii)Bleaching powder used as a bleaching agent in textile industries after dissolving in water.
(iv)Bleaching powder is used as disinfectant in many industries due to its oxidizing property.
(v)Bleaching powder is synthesized by the reaction of chlorine gas with dry slaked lime (calcium hydroxide).
\[Ca{{(OH)}_{2}}+C{{l}_{2}}\to CaOC{{l}_{2}}+{{H}_{2}}O\]
In the above reaction calcium hydroxide reacts with chlorine gas and forms calcium hypochlorite (nothing but Bleaching powder) and water as a side product.
(vi)Bleaching powder is used for disinfecting water to make water suitable for drinking purposes called potable water.
(vii)It is used for bleaching muddy clothes in the laundry.
Note:
Hypochlorite is used in bleaching purposes because it is a very powerful oxidizing agent. Bleaching powder has two types of chlorine atoms. One chlorine has an oxidation number of + 1 and another chlorine atom has oxidation number of -1.
Bleaching powder is called chloride of lime or chlorinated lime.
Complete step by step answer:
(I)Calcium hypochlorite is the chemical name of bleaching powder.
(II)The chemical formula of bleaching powder is \[CaOC{{l}_{2}}\].
(III)The structure of bleaching powder is as follows.

Hence, the correct answer is option D.
Additional Information
(i)Bleaching powder is pale yellow in color and exhibits a strong odor of chlorine.
(ii)Bleaching powder is soluble in water but due to the existence of impurities, we cannot observe a clear solution.
(iii)Bleaching powder used as a bleaching agent in textile industries after dissolving in water.
(iv)Bleaching powder is used as disinfectant in many industries due to its oxidizing property.
(v)Bleaching powder is synthesized by the reaction of chlorine gas with dry slaked lime (calcium hydroxide).
\[Ca{{(OH)}_{2}}+C{{l}_{2}}\to CaOC{{l}_{2}}+{{H}_{2}}O\]
In the above reaction calcium hydroxide reacts with chlorine gas and forms calcium hypochlorite (nothing but Bleaching powder) and water as a side product.
(vi)Bleaching powder is used for disinfecting water to make water suitable for drinking purposes called potable water.
(vii)It is used for bleaching muddy clothes in the laundry.
Note:
Hypochlorite is used in bleaching purposes because it is a very powerful oxidizing agent. Bleaching powder has two types of chlorine atoms. One chlorine has an oxidation number of + 1 and another chlorine atom has oxidation number of -1.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




