
For the reaction\[A \to B + C\], the energy profile diagram is given in the figure.
The decrease in the energy of activation in the presence of catalyst is given by:
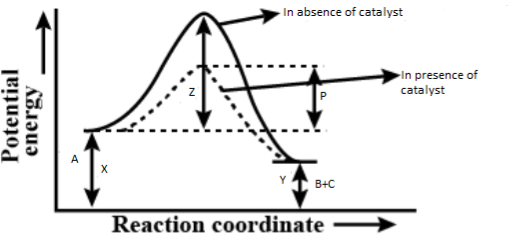
\[
{A:{\text{ }}Z} \\
{B:{\text{ }}Z - P} \\
{C:{\text{ }}Y - Z} \\
{D:{\text{ }}Z - X}
\]

Answer
570k+ views
Hint:The activation energy is referred to as the minimum energy that is required for a chemical reaction to take place. If a reaction is having a low activation energy then that means the reaction will proceed fast because many particles will possess the required energy.
Complete answer:
A Catalyst is basically a substance which is supposed to accelerate the rate of any chemical reaction without requiring to undergo any change in the chemical composition or mass during the chemical reaction. A catalyst accelerates the rate of a chemical reaction by lowering down the activation energy.
It is clearly visible in the figure that in the absence of a catalyst, ‘Z’ is the activation energy while in the presence of the catalyst, ‘P’ is the activation energy. And you can easily observe that \[Z{\text{ }} > {\text{ }}P\]. So, the
So, the decrease in the energy of activation in the presence of a catalyst is given by \[Z - P\].
Hence, the correct answer is Option B.
Note:
Always remember that the activation energy in a reaction is always positive. We know that the energy changes which result from a chemical reaction can either be positive, negative, or even zero, but it is also true that in all of the cases, an energy barrier has to be overcome before any reaction takes place. It should be noted that higher the activation energy, slower will be the chemical reaction.
Complete answer:
A Catalyst is basically a substance which is supposed to accelerate the rate of any chemical reaction without requiring to undergo any change in the chemical composition or mass during the chemical reaction. A catalyst accelerates the rate of a chemical reaction by lowering down the activation energy.
It is clearly visible in the figure that in the absence of a catalyst, ‘Z’ is the activation energy while in the presence of the catalyst, ‘P’ is the activation energy. And you can easily observe that \[Z{\text{ }} > {\text{ }}P\]. So, the
So, the decrease in the energy of activation in the presence of a catalyst is given by \[Z - P\].

Hence, the correct answer is Option B.
Note:
Always remember that the activation energy in a reaction is always positive. We know that the energy changes which result from a chemical reaction can either be positive, negative, or even zero, but it is also true that in all of the cases, an energy barrier has to be overcome before any reaction takes place. It should be noted that higher the activation energy, slower will be the chemical reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




