
The heat of hydrogenation of benzene is \[51\,kcalmo{l^{ - 1}}\] and its resonance energy is \[36\,kcalmo{l^{ - 1}}\]. What will be the heat of hydrogenation of cyclohexene?
A.\[18\,kcalmo{l^{ - 1}}\]
B.\[29\,kcalmo{l^{ - 1}}\]
C.\[50\,kcalmo{l^{ - 1}}\]
D.\[26\,kcalmo{l^{ - 1}}\]
Answer
522.3k+ views
Hint: Heat of hydrogenation: It is the amount of heat required by an unsaturated compound to get converted into a saturated compound by the addition of excess hydrogen. It is always exothermic in nature and represents the strength of carbon-carbon double bond in an alkene.
Complete answer:
Given data in the question:
Heat of hydrogenation of benzene \[ = 51\,kcalmo{l^{ - 1}}\]
Resonance energy of benzene \[ = 36\,kcalmo{l^{ - 1}}\]
As we know, there are total of three double bonds in a benzene i.e., \[{C_6}{H_6}\] molecule, so three moles of hydrogen i.e., \[{H_2}\] are required to convert it into cyclohexane \[({C_6}{H_{12}})\]. The reaction proceeds as follows:
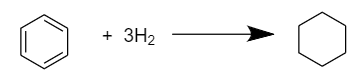
As the double bonds in the benzene ring are in conjugation and show resonance so, the actual heat evolved in the hydrogenation of benzene to form cyclohexane will be equal to the sum of heat of hydrogenation of benzene and its resonance energy.
Actual Heat of hydrogenation \[ = 51 + 36\]
\[ \Rightarrow \Delta {H_{hyd}} = 87kcalmo{l^{ - 1}}\]
Now, to calculate the heat of hydrogenation for cyclohexene \[({C_6}{H_{10}})\], we need to reduce only one double bond i.e., only one mole of hydrogen is required to convert it into cyclohexane\[({C_6}{H_{12}})\]. The reaction proceeds as follows:
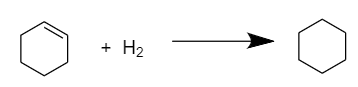
Therefore,
heat of hydrogenation of cyclohexene\[ = \dfrac{{{\text{heat of hydrogenation to reduce three double bonds}}}}{3}\]
\[ \Rightarrow \Delta {H_{hyd}} = \dfrac{{87}}{3}\]
\[ \Rightarrow \Delta {H_{hyd}} = 29kcalmo{l^{ - 1}}\]
Hence, the value of heat of hydrogenation of cyclohexene is \[29kcalmo{l^{ - 1}}\]. Thus from the given options, the option (B) is the correct answer.
Note:
Resonance energy: It is the amount of extra energy that a molecule gains due to extra stability experienced by the presence of a conjugated double bond system. Therefore, while calculating the heat of hydrogenation of a compound that consists of resonance, the resonance energy should be added to the expected value of enthalpy of hydrogenation.
Complete answer:
Given data in the question:
Heat of hydrogenation of benzene \[ = 51\,kcalmo{l^{ - 1}}\]
Resonance energy of benzene \[ = 36\,kcalmo{l^{ - 1}}\]
As we know, there are total of three double bonds in a benzene i.e., \[{C_6}{H_6}\] molecule, so three moles of hydrogen i.e., \[{H_2}\] are required to convert it into cyclohexane \[({C_6}{H_{12}})\]. The reaction proceeds as follows:
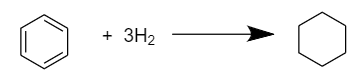
As the double bonds in the benzene ring are in conjugation and show resonance so, the actual heat evolved in the hydrogenation of benzene to form cyclohexane will be equal to the sum of heat of hydrogenation of benzene and its resonance energy.
Actual Heat of hydrogenation \[ = 51 + 36\]
\[ \Rightarrow \Delta {H_{hyd}} = 87kcalmo{l^{ - 1}}\]
Now, to calculate the heat of hydrogenation for cyclohexene \[({C_6}{H_{10}})\], we need to reduce only one double bond i.e., only one mole of hydrogen is required to convert it into cyclohexane\[({C_6}{H_{12}})\]. The reaction proceeds as follows:
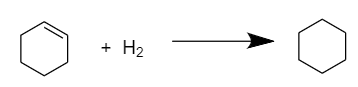
Therefore,
heat of hydrogenation of cyclohexene\[ = \dfrac{{{\text{heat of hydrogenation to reduce three double bonds}}}}{3}\]
\[ \Rightarrow \Delta {H_{hyd}} = \dfrac{{87}}{3}\]
\[ \Rightarrow \Delta {H_{hyd}} = 29kcalmo{l^{ - 1}}\]
Hence, the value of heat of hydrogenation of cyclohexene is \[29kcalmo{l^{ - 1}}\]. Thus from the given options, the option (B) is the correct answer.
Note:
Resonance energy: It is the amount of extra energy that a molecule gains due to extra stability experienced by the presence of a conjugated double bond system. Therefore, while calculating the heat of hydrogenation of a compound that consists of resonance, the resonance energy should be added to the expected value of enthalpy of hydrogenation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




