
For \[{{H}_{3}}P{{O}_{3}}\]and \[{{H}_{3}}P{{O}_{4}}\] the correct choice is:
A. \[{{H}_{3}}P{{O}_{3}}\] is dibasic and reducing.
B. \[{{H}_{3}}P{{O}_{3}}\] is dibasic and non-reducing.
C. \[{{H}_{3}}P{{O}_{4}}\] is tribasic and reducing.
D. \[{{H}_{3}}P{{O}_{4}}\] is tribasic and non-reducing.
Answer
598.2k+ views
Hint: Phosphoric acids are strong acids. It has 3 hydrogen atoms but only 2 are isonisable. Thus it is soluble in water.
Complete step by step solution:
Phosphorus acid or phosphonic acid is a colourless deliquescent crystalline solid which is highly acidic in nature. Even though it has 3 hydrogen, only 2 are ionisable. Hence it is dibasic acid and ionises as
\[{{H}_{3}}P{{O}_{3}}\to {{H}^{+}}+{{H}_{2}}PO_{3}^{-}\]
\[{{H}_{3}}PO_{3}^{-}\to {{H}^{+}}+{{H}_{2}}PO_{3}^{2-}\]
The other hydrogen, which does not take part in ionisation, has a reducing nature. Thus \[{{H}_{3}}P{{O}_{3}}\] act as a reducing agent. So the correct answer for the question is (a).
-Phosphorus acid is obtained by hydrolysis of phosphorus trichloride and phosphorus trioxide.
-Phosphorus acid and its salts are strong reducing agents, as they are readily oxidisable to phosphoric acid and phosphates, respectively.
\[HPO_{3}^{2-}+3O{{H}^{-}}\to PO_{4}^{3-}+2{{H}_{2}}O+2{{e}^{-}}\]
- Phosphorus acid has the capability of reducing salts of copper, silver, gold etc., to their respective metals.
-The structure of phosphorus acid is shown as
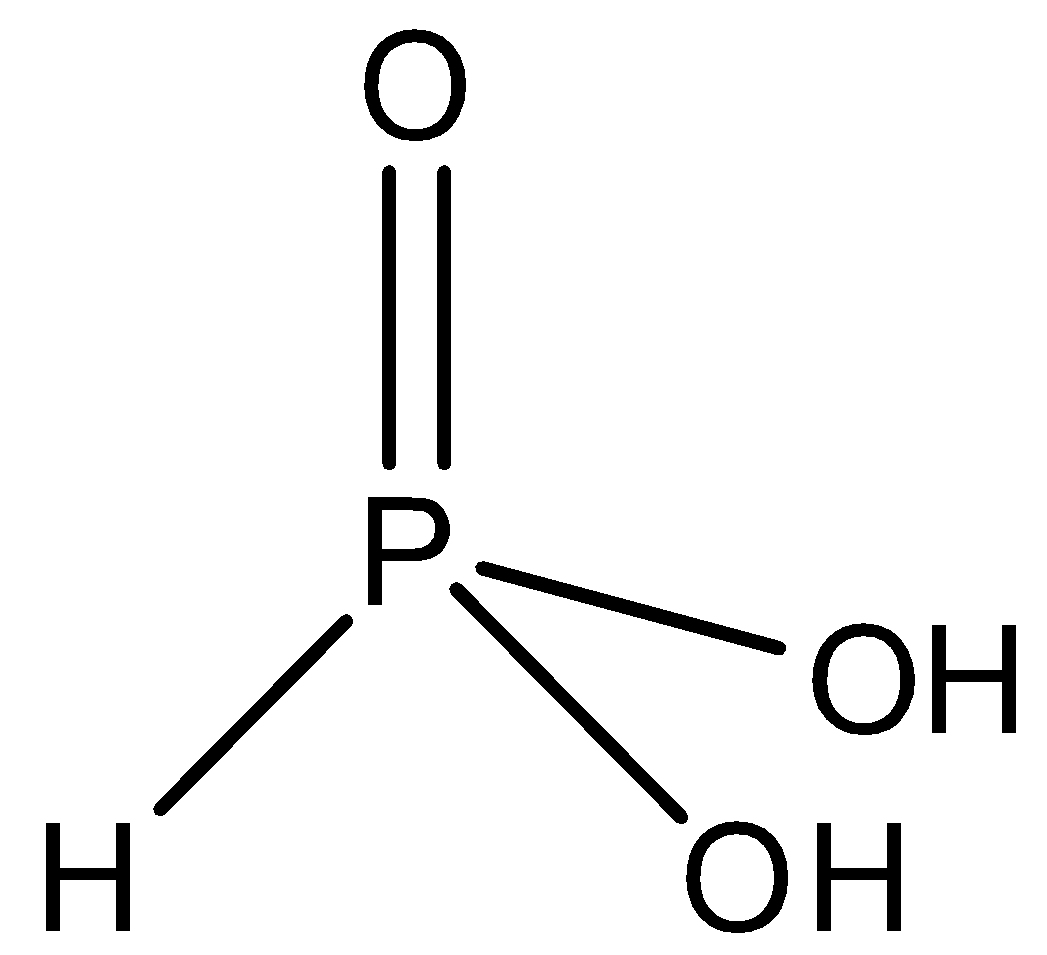
-Orthophosphoric acid \[{{H}_{3}}P{{O}_{4}}\] is a tribasic weak acid. It also has 3 hydrogen atoms, but has 4 oxygen atoms. It is not a reducing agent because all the 3 of its hydrogen ionises in water. It structure is shown as
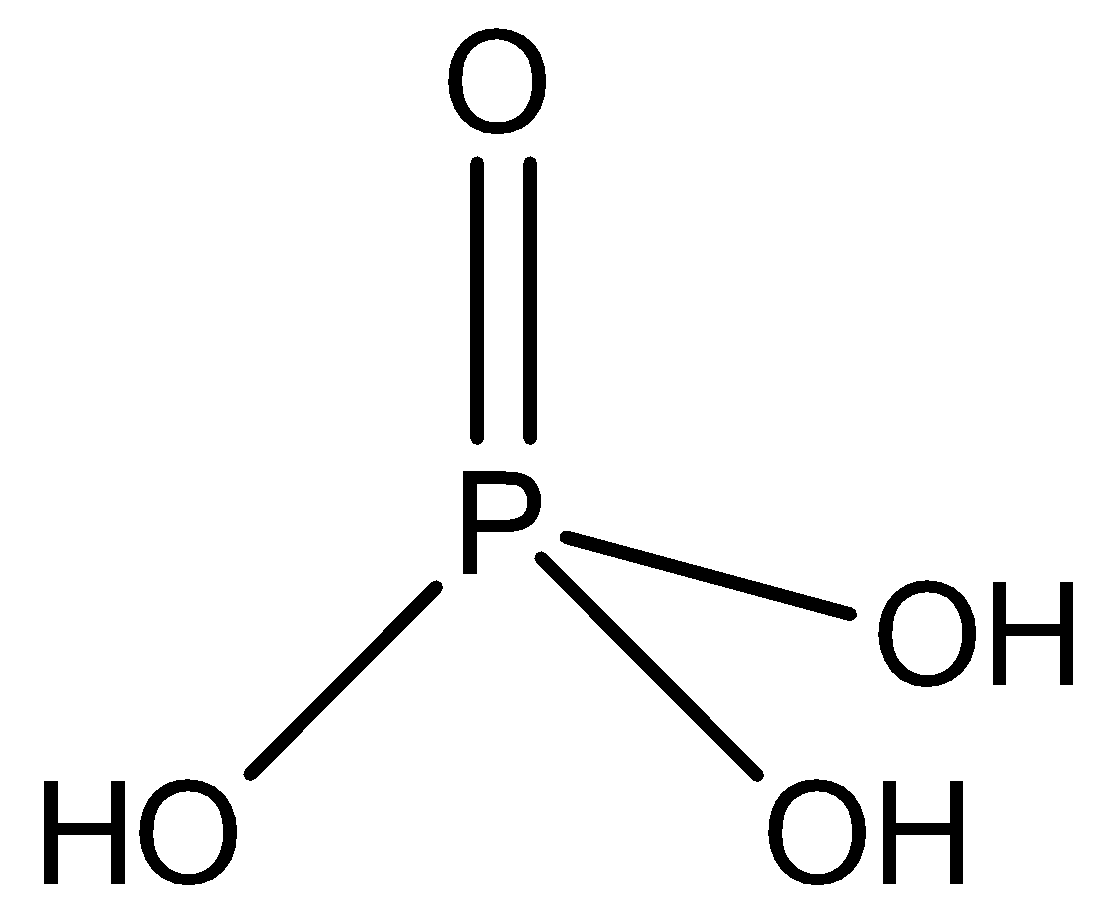
-Phosphoric acid is a syrup liquid which combines with water gives a semi hydrate \[2{{H}_{3}}P{{O}_{4}}.{{H}_{2}}O\]
-It ionises in 3 stages
\[{{H}_{3}}PO_{4}^{{}}\rightleftharpoons {{H}^{+}}+{{H}_{2}}PO_{4}^{-}\]
\[{{H}_{3}}PO_{4}^{-}\rightleftharpoons {{H}^{+}}+{{H}_{2}}PO_{4}^{2-}\]
\[{{H}_{3}}PO_{4}^{2-}\rightleftharpoons {{H}^{+}}+PO_{4}^{3-}\]
Its first ionisation takes place quite fast, but the second and third ionisation takes place very slow. It forms a series of salts.
Thus, the correct answer is option (a), Phosphorus acid is dibasic and reducing.
Note: There are chances that we assume even phosphoric acid is reducing like phosphorus acid. But it is not a reducing agent.
Complete step by step solution:
Phosphorus acid or phosphonic acid is a colourless deliquescent crystalline solid which is highly acidic in nature. Even though it has 3 hydrogen, only 2 are ionisable. Hence it is dibasic acid and ionises as
\[{{H}_{3}}P{{O}_{3}}\to {{H}^{+}}+{{H}_{2}}PO_{3}^{-}\]
\[{{H}_{3}}PO_{3}^{-}\to {{H}^{+}}+{{H}_{2}}PO_{3}^{2-}\]
The other hydrogen, which does not take part in ionisation, has a reducing nature. Thus \[{{H}_{3}}P{{O}_{3}}\] act as a reducing agent. So the correct answer for the question is (a).
-Phosphorus acid is obtained by hydrolysis of phosphorus trichloride and phosphorus trioxide.
-Phosphorus acid and its salts are strong reducing agents, as they are readily oxidisable to phosphoric acid and phosphates, respectively.
\[HPO_{3}^{2-}+3O{{H}^{-}}\to PO_{4}^{3-}+2{{H}_{2}}O+2{{e}^{-}}\]
- Phosphorus acid has the capability of reducing salts of copper, silver, gold etc., to their respective metals.
-The structure of phosphorus acid is shown as

-Orthophosphoric acid \[{{H}_{3}}P{{O}_{4}}\] is a tribasic weak acid. It also has 3 hydrogen atoms, but has 4 oxygen atoms. It is not a reducing agent because all the 3 of its hydrogen ionises in water. It structure is shown as

-Phosphoric acid is a syrup liquid which combines with water gives a semi hydrate \[2{{H}_{3}}P{{O}_{4}}.{{H}_{2}}O\]
-It ionises in 3 stages
\[{{H}_{3}}PO_{4}^{{}}\rightleftharpoons {{H}^{+}}+{{H}_{2}}PO_{4}^{-}\]
\[{{H}_{3}}PO_{4}^{-}\rightleftharpoons {{H}^{+}}+{{H}_{2}}PO_{4}^{2-}\]
\[{{H}_{3}}PO_{4}^{2-}\rightleftharpoons {{H}^{+}}+PO_{4}^{3-}\]
Its first ionisation takes place quite fast, but the second and third ionisation takes place very slow. It forms a series of salts.
Thus, the correct answer is option (a), Phosphorus acid is dibasic and reducing.
Note: There are chances that we assume even phosphoric acid is reducing like phosphorus acid. But it is not a reducing agent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




