
Find x if the ratio of cis isomer to trans isomer is 1: x in \[[M(AA){{b}_{2}}{{c}_{2}}]\].
Answer
594.9k+ views
Hint: When ligands are adjacent to one another in a metal complex, then it is a cis isomer. If the ligands are directly across from one another in the coordination sphere of the metal complex, then it is the trans isomer.
Complete answer:
Geometric isomerism in metal complexes is mainly shown by square planar and octahedral complexes.
\[[M(AA){{b}_{2}}{{c}_{2}}]\] is an octahedral complex and can form geometrical isomers. An example for \[[M(AA){{b}_{2}}{{c}_{2}}]\]type octahedral complex is \[[Co(en){{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]. Here en or ethylene diamine is a bidentate ligand. That is AA in the \[[M(AA){{b}_{2}}{{c}_{2}}]\]refers to a bidentate ligand.
Now the isomers of \[[Co(en){{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\] are
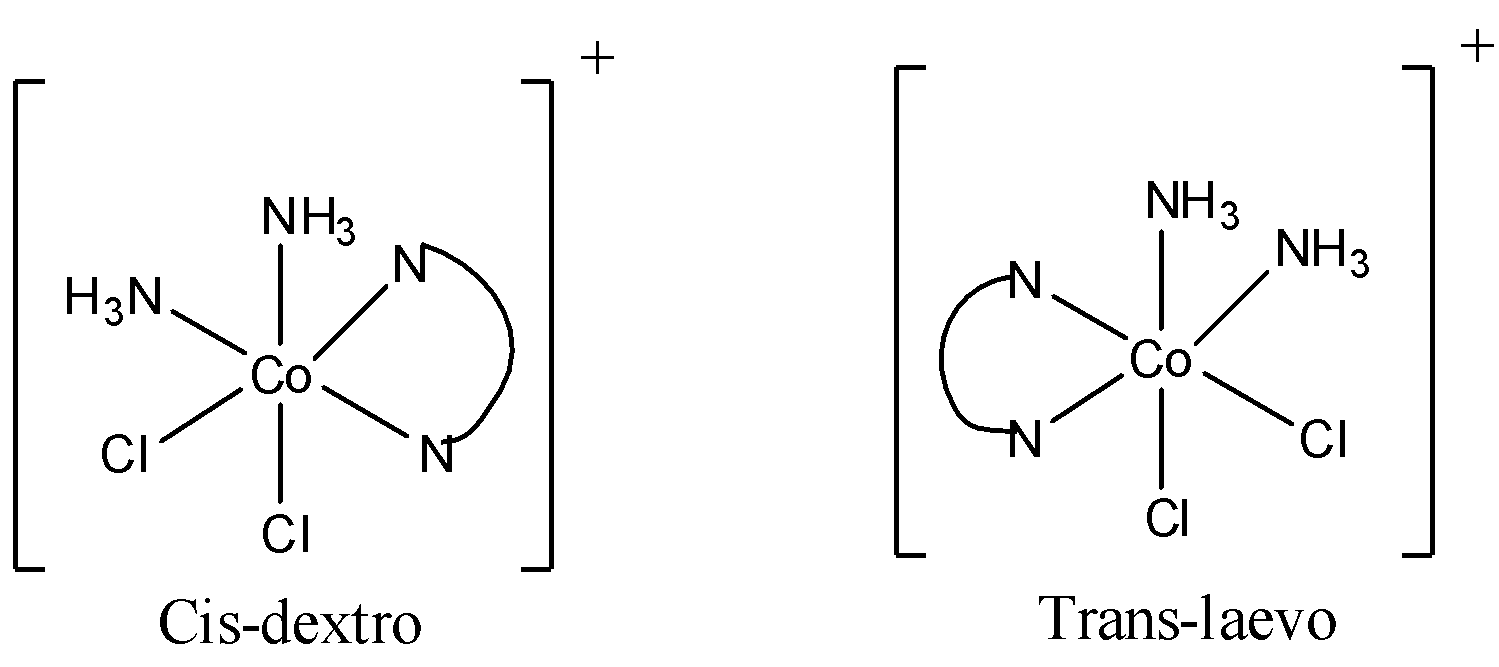
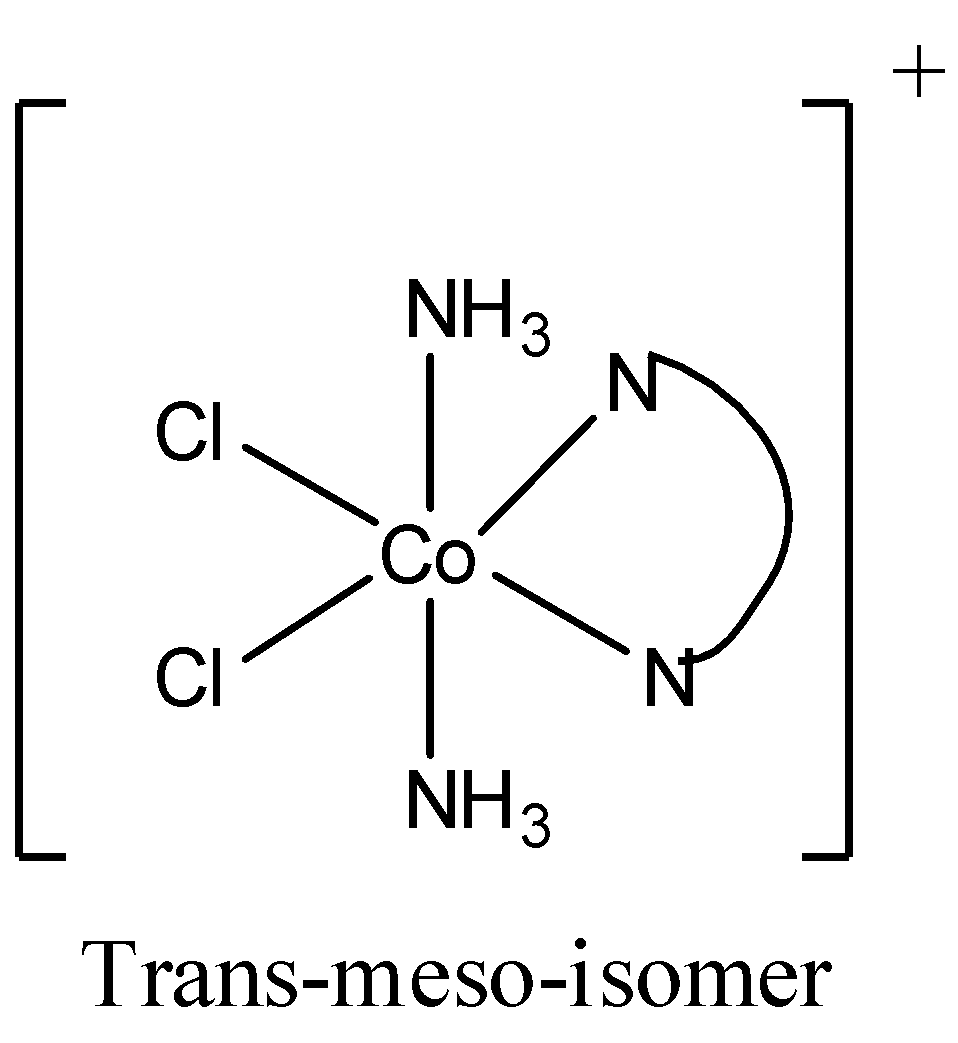
So there are 2 trans isomers, one is trans laevo and other is trans meso (it is optically inactive). Therefore, cis to trans isomer raion is 1:2. So, the x in 1:x is 2.
X=2
Additional Information: Stereoisomers are those which have the same ligands coordinated to the same metal ion and they have different arrangement of ligands in space. These are also called space isomers. Mostly 4-coordinated and 6-coordinated complexes show stereoisomerism. 2-coordinated and 3- coordinated complexes are rare and does not show any stereochemistry because all the ligands are equidistant. 4-coordinated complexes have either square planar or tetrahedral structures. Only square planar complexes show geometrical isomerism. Some types of octahedral complexes show geometrical isomerism.
Note: Stereoisomers are designated as D or dextro and L or leavo according to the direction in which the substance can rotate polarised light. If the polarised light is rotated to the right, then it is D isomer and if the polarised light is rotated to the left, then it is L-isomer. These are optically active. There is meso isomer which is inactive because it can form racemic mixtures.
Complete answer:
Geometric isomerism in metal complexes is mainly shown by square planar and octahedral complexes.
\[[M(AA){{b}_{2}}{{c}_{2}}]\] is an octahedral complex and can form geometrical isomers. An example for \[[M(AA){{b}_{2}}{{c}_{2}}]\]type octahedral complex is \[[Co(en){{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\]. Here en or ethylene diamine is a bidentate ligand. That is AA in the \[[M(AA){{b}_{2}}{{c}_{2}}]\]refers to a bidentate ligand.
Now the isomers of \[[Co(en){{(N{{H}_{3}})}_{2}}C{{l}_{2}}]\] are


So there are 2 trans isomers, one is trans laevo and other is trans meso (it is optically inactive). Therefore, cis to trans isomer raion is 1:2. So, the x in 1:x is 2.
X=2
Additional Information: Stereoisomers are those which have the same ligands coordinated to the same metal ion and they have different arrangement of ligands in space. These are also called space isomers. Mostly 4-coordinated and 6-coordinated complexes show stereoisomerism. 2-coordinated and 3- coordinated complexes are rare and does not show any stereochemistry because all the ligands are equidistant. 4-coordinated complexes have either square planar or tetrahedral structures. Only square planar complexes show geometrical isomerism. Some types of octahedral complexes show geometrical isomerism.
Note: Stereoisomers are designated as D or dextro and L or leavo according to the direction in which the substance can rotate polarised light. If the polarised light is rotated to the right, then it is D isomer and if the polarised light is rotated to the left, then it is L-isomer. These are optically active. There is meso isomer which is inactive because it can form racemic mixtures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




