
Find the correct option for product ${{Y}}$ .
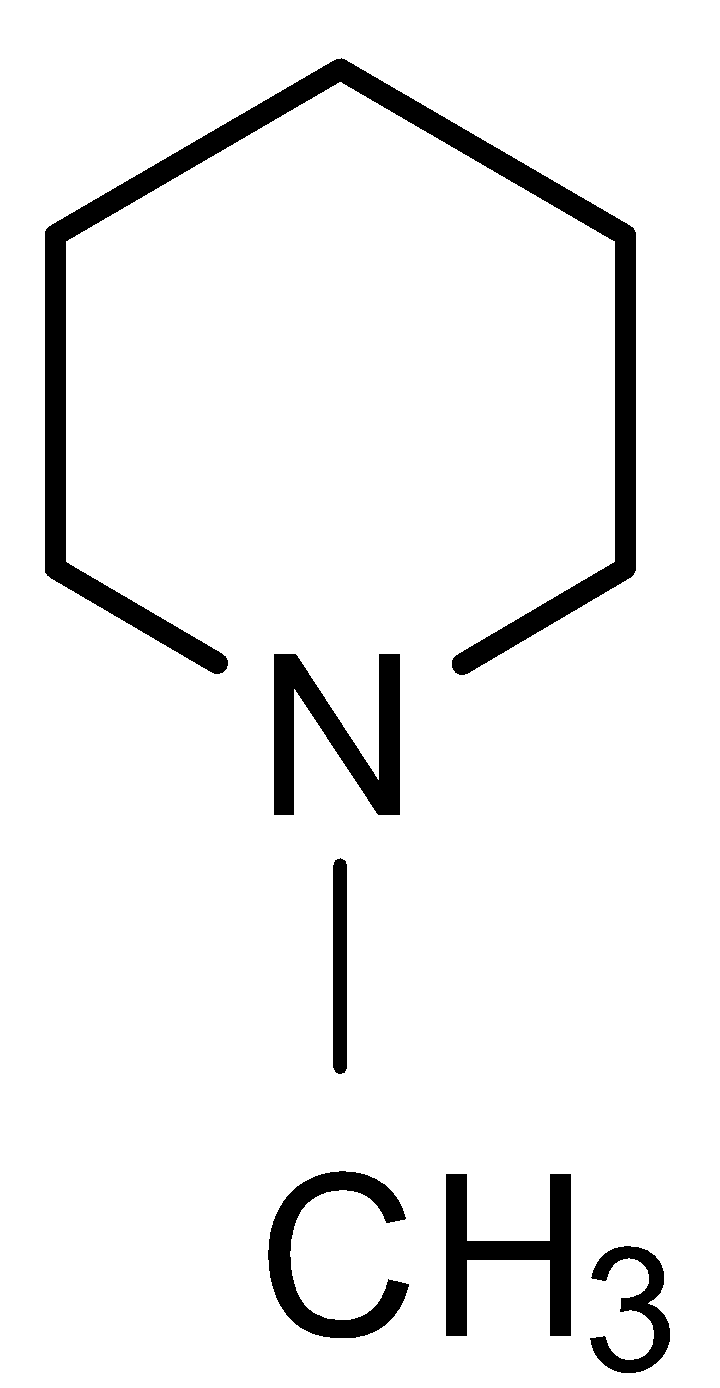 $\xrightarrow{{{{C}}{{{H}}_3}{{I (excess)}}}}$ ${{X}}$ $\xrightarrow{{{{Moist A}}{{{g}}_2}{{O / }}\Delta }}$ ${{Y}}$ .
$\xrightarrow{{{{C}}{{{H}}_3}{{I (excess)}}}}$ ${{X}}$ $\xrightarrow{{{{Moist A}}{{{g}}_2}{{O / }}\Delta }}$ ${{Y}}$ .
Find the correct option for product ${{Y}}$ .
A)
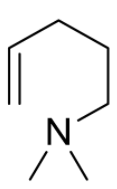
B)
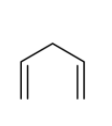
C)
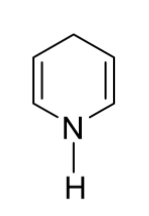
D)
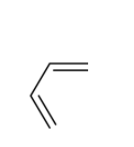





Answer
568.8k+ views
Hint:The starting molecule is a tertiary amine and the first reagent used in the reaction is methyl iodide which suggests the possibility of methylation of the amine. The intermediate ${{X}}$ on treatment with silver oxide on heating gives the final product. This could possibly suggest the precipitation of silver iodide which can occur if a salt is produced.
Complete step by step answer:
The given reaction undergoes Hofmann exhaustive methylation reaction or Hofmann elimination reaction. It is a method of producing tertiary amines and alkenes by the treatment of amines with excess amounts of methyl iodide. The major product obtained in the course of this reaction is a least substituted and least stable alkene which contrasts the idea of Zaitsev rule which states that the most substituted product will be the most stable and the most favoured product.
Mechanism of Hofmann elimination reaction is as follows:
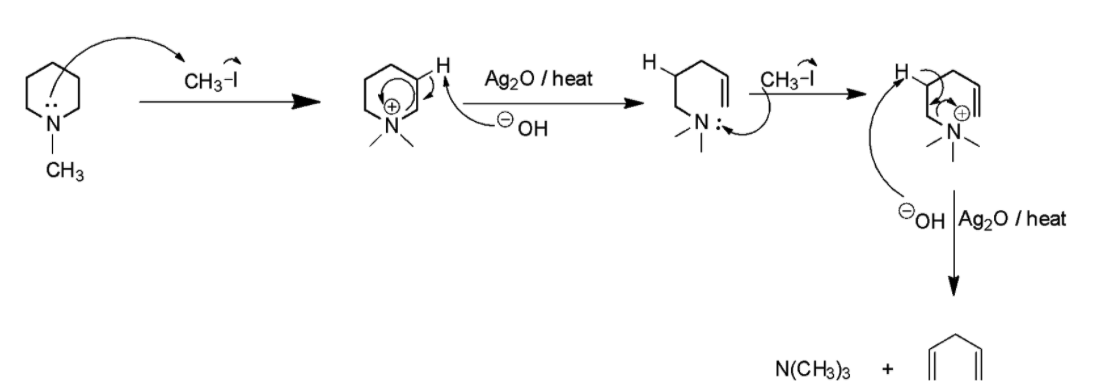
The mechanism of the reaction starts with the lone pair of electrons on amine attacking the methyl iodide which already has a partial positive charge on methyl and partial negative on iodine. The methyl attaches to the tertiary amine to give a quaternary ammonium salt. The obtained intermediate on exposure to ${{A}}{{{g}}_2}{{O}}$ in aqueous medium produces hydroxyl ions. Since, hydroxyl ions are very good bases, they try to abstract the beta-hydrogen from the quaternary ammonium salt. When they combine, it produces a neutral water molecule, which gets eliminated out of the quaternary ammonium salt. The free bond which contains two electrons now shifts to form a pi-bond, leading to a bond breakage. The broken bond gives its electrons to the positively charged nitrogen in the quaternary ammonium salt so that the lone pair of electrons on nitrogen is recovered. Now, since there is excess methyl iodide in the solution, the same event happens again with the methyl group getting attached to the nitrogen by taking its lone pair of electrons from nitrogen leading to the formation of quaternary ammonium salt again. Again, it reacts with ${{A}}{{{g}}_2}{{O}}$ in aqueous medium inducing a beta elimination of hydrogen leading to the formation of a double bond. The major product and side product obtained in this reaction are penta $ - 1,4 - $ diene or $1,4 - $ pentadiene and a tertiary amine.
Hence, option (B) is the correct answer.
Note:
For Hofmann reactions to occur, amine containing an alkyl group with a beta hydrogen only should be taken, otherwise the required alkene major product by elimination, which is the significant part of the reaction will not be possible. When a given alkyl group contains two different sets of beta hydrogens, make sure that the major product is the less substituted one.
Complete step by step answer:
The given reaction undergoes Hofmann exhaustive methylation reaction or Hofmann elimination reaction. It is a method of producing tertiary amines and alkenes by the treatment of amines with excess amounts of methyl iodide. The major product obtained in the course of this reaction is a least substituted and least stable alkene which contrasts the idea of Zaitsev rule which states that the most substituted product will be the most stable and the most favoured product.
Mechanism of Hofmann elimination reaction is as follows:

The mechanism of the reaction starts with the lone pair of electrons on amine attacking the methyl iodide which already has a partial positive charge on methyl and partial negative on iodine. The methyl attaches to the tertiary amine to give a quaternary ammonium salt. The obtained intermediate on exposure to ${{A}}{{{g}}_2}{{O}}$ in aqueous medium produces hydroxyl ions. Since, hydroxyl ions are very good bases, they try to abstract the beta-hydrogen from the quaternary ammonium salt. When they combine, it produces a neutral water molecule, which gets eliminated out of the quaternary ammonium salt. The free bond which contains two electrons now shifts to form a pi-bond, leading to a bond breakage. The broken bond gives its electrons to the positively charged nitrogen in the quaternary ammonium salt so that the lone pair of electrons on nitrogen is recovered. Now, since there is excess methyl iodide in the solution, the same event happens again with the methyl group getting attached to the nitrogen by taking its lone pair of electrons from nitrogen leading to the formation of quaternary ammonium salt again. Again, it reacts with ${{A}}{{{g}}_2}{{O}}$ in aqueous medium inducing a beta elimination of hydrogen leading to the formation of a double bond. The major product and side product obtained in this reaction are penta $ - 1,4 - $ diene or $1,4 - $ pentadiene and a tertiary amine.
Hence, option (B) is the correct answer.
Note:
For Hofmann reactions to occur, amine containing an alkyl group with a beta hydrogen only should be taken, otherwise the required alkene major product by elimination, which is the significant part of the reaction will not be possible. When a given alkyl group contains two different sets of beta hydrogens, make sure that the major product is the less substituted one.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




