
Explain why boiling points of carboxylic acids are higher than corresponding alcohols.
Answer
588k+ views
Hint: Carboxylic acid is made up of the carbonyl group $\text{(-C=O)}$ and the hydroxyl group$\text{-OH}$. The carboxylic acid may be aliphatic $\text{R-COOH}$ or aromatic $\text{Ar-COOH}$ depending upon the attachment of the group to the aliphatic or aryl group respectively.
Complete answer:
> The carboxylic acid has a higher boiling point. The higher boiling point is due to the presence of intermolecular hydrogen bonding. The hydrogen bond is the stronger bond between the electronegative atoms like $\text{O,S,F,etc}$ between the $\text{H}$ atoms.
> In carboxylic acid, the hydrogen bonding is established between the hydrogen of one carboxylic acid and the oxygen of the carbonyl group of the other carboxylic acid.
> Due to hydrogen bonding, the carboxylic acid exists as dimers. The hydrogen bonds are not broken easily and completely even in the vapour phase. Thus they require higher heat energy to break or separate the molecules from each other.
> On comparing it with the corresponding alcohol, carboxylic acid has a higher boiling than the corresponding aldehyde, ketone, and even alcohols of the comparable molecular masses.
Let's take an example of ethanoic acid and propanol. They have the same molecular mass 60. The boiling point of the ethanoic acid is $\text{391K}$ whereas that of the propanol is $\text{370K}$.
The higher boiling points of carboxylic acid compared to alcohol is due to the following reasons:
(i) As compared to alcohols, the $\text{O-H}$ bond in carboxylic acids is more strongly polarised due to the presence of adjacent electron-withdrawing carbonyl groups. Therefore, carboxylic acids can form a stronger hydrogen bond.
(ii) The molecules of carboxylic acids are held together by two hydrogen bonds and therefore, form cyclic dimers.
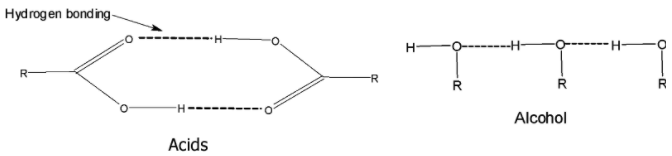
We can say that the carboxylic acid molecules are held together by the strong attractive forces and therefore, they have a boiling point.
Such types of attractive forces do not exist between the alcohols. Alcohols do have the hydrogen bonding in it. But the hydrogen bonding is not as strong as that of the carboxylic acid.
Note: Remember that the boiling point is the heat energy required to separate the molecules. The boiling point is directly proportional to the molecular mass. Hydrogen and oxygen forms a hydrogen bonding. Hydrogen bonding is the reason behind the various properties.
Complete answer:
> The carboxylic acid has a higher boiling point. The higher boiling point is due to the presence of intermolecular hydrogen bonding. The hydrogen bond is the stronger bond between the electronegative atoms like $\text{O,S,F,etc}$ between the $\text{H}$ atoms.
> In carboxylic acid, the hydrogen bonding is established between the hydrogen of one carboxylic acid and the oxygen of the carbonyl group of the other carboxylic acid.
> Due to hydrogen bonding, the carboxylic acid exists as dimers. The hydrogen bonds are not broken easily and completely even in the vapour phase. Thus they require higher heat energy to break or separate the molecules from each other.
> On comparing it with the corresponding alcohol, carboxylic acid has a higher boiling than the corresponding aldehyde, ketone, and even alcohols of the comparable molecular masses.
Let's take an example of ethanoic acid and propanol. They have the same molecular mass 60. The boiling point of the ethanoic acid is $\text{391K}$ whereas that of the propanol is $\text{370K}$.
The higher boiling points of carboxylic acid compared to alcohol is due to the following reasons:
(i) As compared to alcohols, the $\text{O-H}$ bond in carboxylic acids is more strongly polarised due to the presence of adjacent electron-withdrawing carbonyl groups. Therefore, carboxylic acids can form a stronger hydrogen bond.
(ii) The molecules of carboxylic acids are held together by two hydrogen bonds and therefore, form cyclic dimers.

We can say that the carboxylic acid molecules are held together by the strong attractive forces and therefore, they have a boiling point.
Such types of attractive forces do not exist between the alcohols. Alcohols do have the hydrogen bonding in it. But the hydrogen bonding is not as strong as that of the carboxylic acid.
Note: Remember that the boiling point is the heat energy required to separate the molecules. The boiling point is directly proportional to the molecular mass. Hydrogen and oxygen forms a hydrogen bonding. Hydrogen bonding is the reason behind the various properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




