
Explain the use of radioactive isotopes in the study of the mechanism of photosynthesis in plants and hydrolysis of esters.
Answer
531k+ views
Hint: The radioactive isotopes are the elements having unstable nuclei and can be used to study reaction mechanisms. One radioactive isotope of oxygen - ${{\text{O}}^{18}}$ is used in a reactant and the products formed in the reaction are studied to confirm the mechanism of reactions such as photosynthesis and hydrolysis of esters.
Complete answer:
Isotopes are the elements having the same number of protons but differ in the number of neutrons. Radioactive isotopes are the elements having an unstable combination of neutrons and protons and it dissipates energy by emitting radiations to attain stability.
Many radioactive isotopes have useful applications in the real world. For example, they are used to study the mechanism of many chemical reactions. Let us discuss how the radioactive isotopes are used to study the reaction mechanism of photosynthesis and hydrolysis of esters.
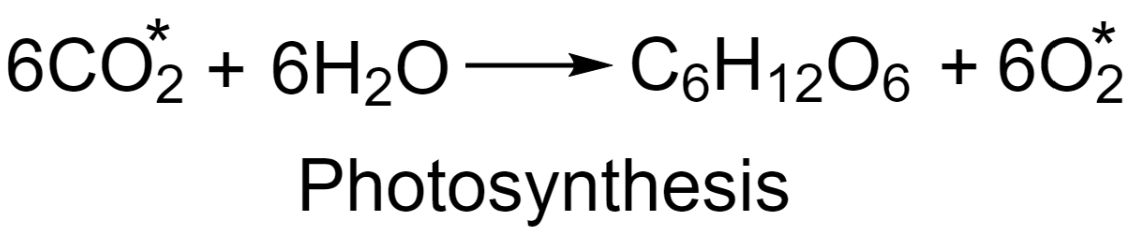
-Study of the reaction mechanism of photosynthesis:
In this method, a small quantity of carbon dioxide having ${{\text{O}}^{18}}$ a radioactive isotope of oxygen is mixed with ordinary $\text{C}{{\text{O}}_{2}}$ and the process of photosynthesis is being carried out. The results reveal that the oxygen produced in the reaction is non-radioactive. This indicates that the mechanism followed in photosynthesis involves the production of oxygen from water and not from carbon dioxide.
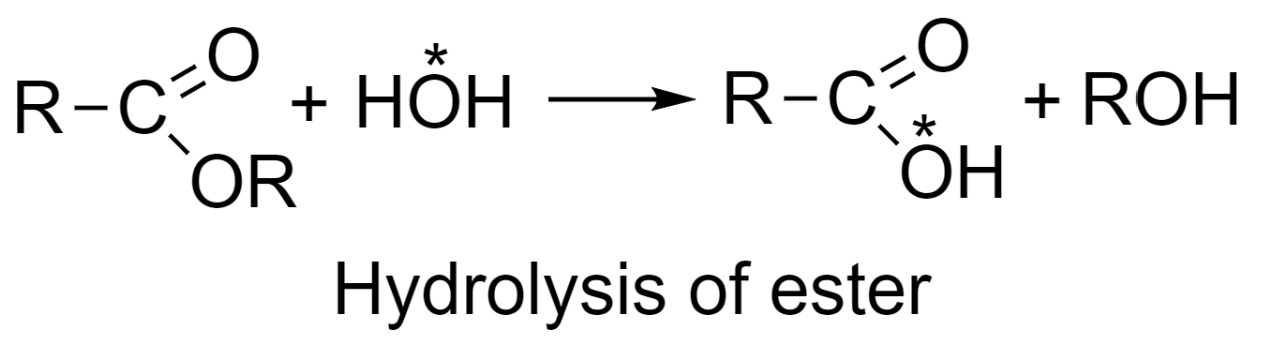
-Study of the reaction mechanism of ester hydrolysis:
Similar to the method explained above, in the hydrolysis of esters, water used is containing ${{\text{O}}^{18}}$ radioactive isotope and the products formed in the reaction are alcohol and an acid possessing radioactivity. Thus, the mechanism of ester hydrolysis would be as given in the following reaction in which OH group of water is retained in acid formed:
Note:
There are many radioactive isotopes of oxygen ranging from ${{\text{O}}^{11}}$ to ${{\text{O}}^{26}}$. But these are short-lived species and out of all these, the most stable known isotopes of oxygen that are used in real-life applications are - ${{\text{O}}^{16}},\text{ }{{\text{O}}^{17}},\text{ }{{\text{O}}^{18}}$.
Complete answer:
Isotopes are the elements having the same number of protons but differ in the number of neutrons. Radioactive isotopes are the elements having an unstable combination of neutrons and protons and it dissipates energy by emitting radiations to attain stability.
Many radioactive isotopes have useful applications in the real world. For example, they are used to study the mechanism of many chemical reactions. Let us discuss how the radioactive isotopes are used to study the reaction mechanism of photosynthesis and hydrolysis of esters.
-Study of the reaction mechanism of photosynthesis:
In this method, a small quantity of carbon dioxide having ${{\text{O}}^{18}}$ a radioactive isotope of oxygen is mixed with ordinary $\text{C}{{\text{O}}_{2}}$ and the process of photosynthesis is being carried out. The results reveal that the oxygen produced in the reaction is non-radioactive. This indicates that the mechanism followed in photosynthesis involves the production of oxygen from water and not from carbon dioxide.

-Study of the reaction mechanism of ester hydrolysis:
Similar to the method explained above, in the hydrolysis of esters, water used is containing ${{\text{O}}^{18}}$ radioactive isotope and the products formed in the reaction are alcohol and an acid possessing radioactivity. Thus, the mechanism of ester hydrolysis would be as given in the following reaction in which OH group of water is retained in acid formed:

Note:
There are many radioactive isotopes of oxygen ranging from ${{\text{O}}^{11}}$ to ${{\text{O}}^{26}}$. But these are short-lived species and out of all these, the most stable known isotopes of oxygen that are used in real-life applications are - ${{\text{O}}^{16}},\text{ }{{\text{O}}^{17}},\text{ }{{\text{O}}^{18}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




