
How can I explain the mechanism of acid-catalyzed epoxidation of alkenes with peroxy acids$?$
Answer
526.8k+ views
Hint: Acid catalyzed hydration of alkenes involves replacing the pi bond on an alkene with a water molecule. This is done by adding alcohol to the more substituted carbon atom, and hydrogen to the less substituted carbon atom. This follows markoVnikov’s rule.
Complete step by step solution:
Step $1$: protonation of alkene to form carbocation by electrophilic attack of ${H_3}{O^ + }$.
According to Markovnikov's Rule, the proton adds to the more subbed carbon ions departing the less subbed carbon inadequate. With just three bonds and a vacant p orbital = a carbocation structures. Look out for a potential carbocation rearrangement, most notably when a secondary carbocation is adjacent to a tertiary or quaternary carbon. Since the carbocation is best in arrangement (the new electrophile), and will pull in the consideration of a close by water particle, especially the part of the way negative oxygen molecule with its solitary pair of electrons. Oxygen utilizes its electrons to assault the carbocation. This structures a connection among itself and carbon.

Step $2$: Nucleophillic attack of water of carbocation.
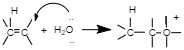
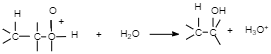
Step $3$: Deprotonation to form an alcohol.
The last step of this reaction is the removal of the additional H molecule to yield an neutral alcohol. A will show the form base of the underlying acidic catalyst pulling the proton. Others will show a dissolvable atom pulling the proton. Still others will show B for 'base' suggesting that anything can pull off the last proton.
Note:
When utilizing a strong acid, the acid separation in arrangement which structures hydronium is programmed and frequently underestimated. Remember that protons don’t float around freely in solution. Instead they are picked up by a solvent molecule, in this case water, to form a protonated solvent molecule.
Complete step by step solution:
Step $1$: protonation of alkene to form carbocation by electrophilic attack of ${H_3}{O^ + }$.
According to Markovnikov's Rule, the proton adds to the more subbed carbon ions departing the less subbed carbon inadequate. With just three bonds and a vacant p orbital = a carbocation structures. Look out for a potential carbocation rearrangement, most notably when a secondary carbocation is adjacent to a tertiary or quaternary carbon. Since the carbocation is best in arrangement (the new electrophile), and will pull in the consideration of a close by water particle, especially the part of the way negative oxygen molecule with its solitary pair of electrons. Oxygen utilizes its electrons to assault the carbocation. This structures a connection among itself and carbon.

Step $2$: Nucleophillic attack of water of carbocation.

Step $3$: Deprotonation to form an alcohol.
The last step of this reaction is the removal of the additional H molecule to yield an neutral alcohol. A will show the form base of the underlying acidic catalyst pulling the proton. Others will show a dissolvable atom pulling the proton. Still others will show B for 'base' suggesting that anything can pull off the last proton.

Note:
When utilizing a strong acid, the acid separation in arrangement which structures hydronium is programmed and frequently underestimated. Remember that protons don’t float around freely in solution. Instead they are picked up by a solvent molecule, in this case water, to form a protonated solvent molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




