
Explain the following with suitable examples
(i).Clemmensen Reduction
(ii).Stephen reaction
Answer
518.7k+ views
Hint: The Clemmensen reduction is a reaction that uses hydrochloric acid and zinc amalgam to convert aldehydes or ketones to alkanes. The Clemmensen reduction is named after Erik Christian Clemmensen, a Danish chemist.
In Stephen reaction, When nitrile is reduced with stannous chloride and hydrogen chloride steam, an imine intermediate is formed, as seen above (in ethyl acetate solvent). The resulting aldehyde is obtained by hydrolysis of this imine intermediate.
Complete answer:
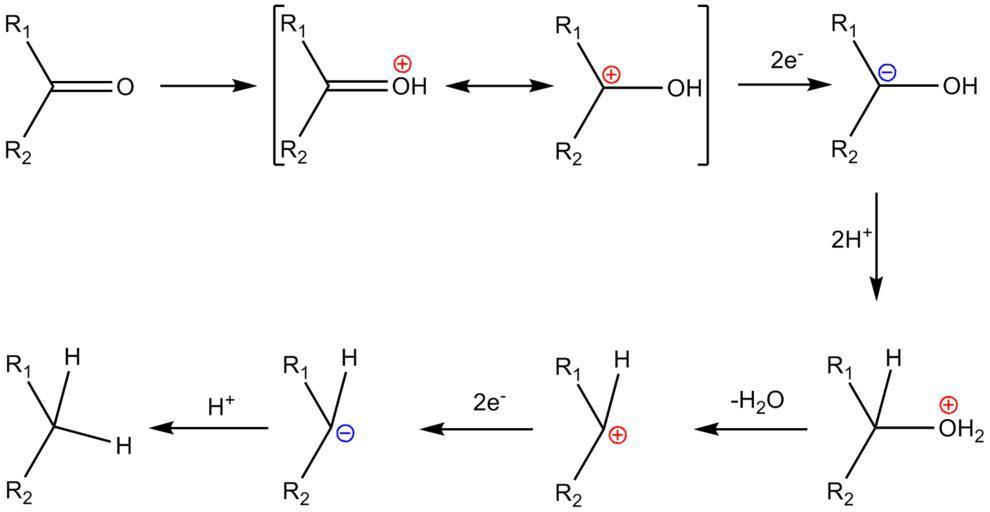
Clemmensen Reduction
The Clemmensen reduction is a reaction that uses hydrochloric acid and zinc amalgam to convert aldehydes or ketones to alkanes. The Clemmensen reduction is named after Erik Christian Clemmensen, a Danish chemist.
This reaction is especially useful for reducing aryl-alkyl ketones generated by Friedel-Crafts acylation. In the reduction of cyclic ketones or aliphatic and zinc metal, the reaction is more successful.
Stephen Reaction
The Addition of Gaseous Hydrogen Chloride to the Given Nitrile Starts the Stephen Reaction Mechanism. Henry Stephen, the reaction's inventor, was given the name Stephen aldehyde synthesis. The reaction entails the formation of aldehydes from nitriles using tin(II) chloride and hydrochloric acid, followed by the quenching of the iminium salt with water. Another useful byproduct of this reaction is ammonium chloride.
The Stephan reaction is used to generate acetaldehyde from methyl cyanide, as seen in the diagram below.
\[C{H_3}CN + 2{H^ + }\xrightarrow[{HCl}]{{SnC{l_2}}}C{H_3} - CH - NH\xrightarrow{{{H_2}O}}N{H_3} + C{H_3}CHO\]
When nitrile is reduced with stannous chloride and hydrogen chloride steam, an imine intermediate is formed, as seen above (in ethyl acetate solvent). The resulting aldehyde is obtained by hydrolysis of this imine intermediate.
Note:
The Clemmensen reduction is a reaction that uses hydrochloric acid and zinc amalgam to convert aldehydes or ketones to alkanes. The Clemmensen reduction is named after Erik Christian Clemmensen, a Danish chemist.
In Stephen reaction, When nitrile is reduced with stannous chloride and hydrogen chloride steam, an imine intermediate is formed, as seen above (in ethyl acetate solvent). The resulting aldehyde is obtained by hydrolysis of this imine intermediate.
In Stephen reaction, When nitrile is reduced with stannous chloride and hydrogen chloride steam, an imine intermediate is formed, as seen above (in ethyl acetate solvent). The resulting aldehyde is obtained by hydrolysis of this imine intermediate.
Complete answer:
Clemmensen Reduction
The Clemmensen reduction is a reaction that uses hydrochloric acid and zinc amalgam to convert aldehydes or ketones to alkanes. The Clemmensen reduction is named after Erik Christian Clemmensen, a Danish chemist.
This reaction is especially useful for reducing aryl-alkyl ketones generated by Friedel-Crafts acylation. In the reduction of cyclic ketones or aliphatic and zinc metal, the reaction is more successful.

Stephen Reaction
The Addition of Gaseous Hydrogen Chloride to the Given Nitrile Starts the Stephen Reaction Mechanism. Henry Stephen, the reaction's inventor, was given the name Stephen aldehyde synthesis. The reaction entails the formation of aldehydes from nitriles using tin(II) chloride and hydrochloric acid, followed by the quenching of the iminium salt with water. Another useful byproduct of this reaction is ammonium chloride.
The Stephan reaction is used to generate acetaldehyde from methyl cyanide, as seen in the diagram below.
\[C{H_3}CN + 2{H^ + }\xrightarrow[{HCl}]{{SnC{l_2}}}C{H_3} - CH - NH\xrightarrow{{{H_2}O}}N{H_3} + C{H_3}CHO\]
When nitrile is reduced with stannous chloride and hydrogen chloride steam, an imine intermediate is formed, as seen above (in ethyl acetate solvent). The resulting aldehyde is obtained by hydrolysis of this imine intermediate.
Note:
The Clemmensen reduction is a reaction that uses hydrochloric acid and zinc amalgam to convert aldehydes or ketones to alkanes. The Clemmensen reduction is named after Erik Christian Clemmensen, a Danish chemist.
In Stephen reaction, When nitrile is reduced with stannous chloride and hydrogen chloride steam, an imine intermediate is formed, as seen above (in ethyl acetate solvent). The resulting aldehyde is obtained by hydrolysis of this imine intermediate.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




