
Ethylene gives epoxy ethane on oxidation with
(A) $ KMn{O_4}\,\,and\,\,O{H^ - } $
(B) $ {K_2}C{r_2}{O_7}\,and\,{H^ + } $
(C) $ Ag\,\,at\,\,{200^ \circ }C $
(D) $ {H_2}S{O_4}\,\,at\,\,{170^ \circ }C $
Answer
520.8k+ views
Hint :Epoxy ethane, also known as oxirane and ethylene oxide, is a very reactive cyclic ether with an oxygen atom attached to carbon atoms in an equilateral triangle. It is a colorless, non polar, volatile and flammable chemical compound. Oxidation of ethylene yields ethylene oxide.
Complete Step By Step Answer:
The IUPAC name of ethylene is ethane. It contains two $ s{p^2} $ hybridised carbon atoms with a double bond. Reactivity of ethylene is due to the $ \pi $ bond and is the center for electrophilic reactions. Epoxy ethane is formed by the oxidation of double bonds in ethane. Now, let us discuss the above given chemical reactions.
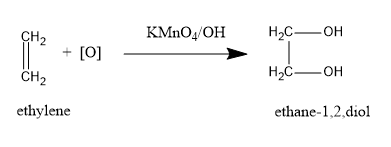
(A)Potassium permanganate in an alkaline medium is a strong oxidising agent. It oxidises the double bond in alkene giving vicinal diols. Ethylene on oxidation with alkaline $ KMn{O_4} $ undergoes hydroxylation reaction and yields ethylene glycol or ethane $ - 1,2 - $ diol. The solution colour changes from pink ( $ KMn{O_4} $ ) to brown ( $ Mn{O_2} $ ).
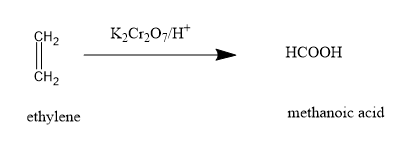
(B)Acidic potassium dichromate is not a strong oxidising agent as alkaline potassium permanganate. Oxidation of alkenes with it gives ketones or carboxylic acids. The reaction with ethylene gives:
Further oxidation gives carbon dioxide and water.
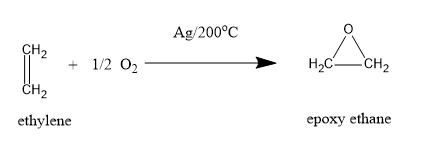
(C)Oxidation reaction of ethylene with Ag is:
When rearrangement of H in oxirane takes place giving acetaldehyde.
(D)Sulphuric acid is not a strong oxidizing agent like the above reagents. Reaction of ethylene with cold sulphuric acid gives ethyl hydrogen sulphate, $ C{H_3}C{H_2}OS{O_3}H $ .
Thus, the answer is (D) $ {H_2}S{O_4}\,\,at\,\,{170^ \circ }C $ .
Note :
Epoxy ethane has a three-atom ring which has ring strain due to which it is highly reactive.
Ethylene is a colourless, inflammable chemical compound. It is an important gaseous plant hormone and regulates growth and development of plants, fastens the ripening of fruits and promotes senescence. It is important in promoting negative geotropism for the growth of roots and absorption of minerals from soil.
Complete Step By Step Answer:
The IUPAC name of ethylene is ethane. It contains two $ s{p^2} $ hybridised carbon atoms with a double bond. Reactivity of ethylene is due to the $ \pi $ bond and is the center for electrophilic reactions. Epoxy ethane is formed by the oxidation of double bonds in ethane. Now, let us discuss the above given chemical reactions.
(A)Potassium permanganate in an alkaline medium is a strong oxidising agent. It oxidises the double bond in alkene giving vicinal diols. Ethylene on oxidation with alkaline $ KMn{O_4} $ undergoes hydroxylation reaction and yields ethylene glycol or ethane $ - 1,2 - $ diol. The solution colour changes from pink ( $ KMn{O_4} $ ) to brown ( $ Mn{O_2} $ ).

(B)Acidic potassium dichromate is not a strong oxidising agent as alkaline potassium permanganate. Oxidation of alkenes with it gives ketones or carboxylic acids. The reaction with ethylene gives:

Further oxidation gives carbon dioxide and water.
(C)Oxidation reaction of ethylene with Ag is:

When rearrangement of H in oxirane takes place giving acetaldehyde.
(D)Sulphuric acid is not a strong oxidizing agent like the above reagents. Reaction of ethylene with cold sulphuric acid gives ethyl hydrogen sulphate, $ C{H_3}C{H_2}OS{O_3}H $ .
Thus, the answer is (D) $ {H_2}S{O_4}\,\,at\,\,{170^ \circ }C $ .
Note :
Epoxy ethane has a three-atom ring which has ring strain due to which it is highly reactive.
Ethylene is a colourless, inflammable chemical compound. It is an important gaseous plant hormone and regulates growth and development of plants, fastens the ripening of fruits and promotes senescence. It is important in promoting negative geotropism for the growth of roots and absorption of minerals from soil.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




