
Ethanol on reaction of acidified potassium dichromate form:
A. ethane
B. ethene
C. ethanal
D. ethanoic acid
Answer
569.1k+ views
Hint:The oxidation of an alcohol forms an aldehyde. It can be further oxidized to carboxylic acid. This conversion usually takes place in presence of strong oxidising agents like $acidified \,KMnO_4,\, K_2Cr_2O_7$ etc.
Complete answer:
The structure of ethanol is as shown below:
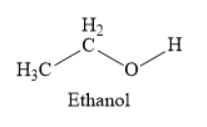
Ethanol is an alcohol. It is also called ethyl alcohol. It contains two carbon atoms as part of an ethyl group and one hydroxyl group.
Acidified potassium dichromate is a strong oxidizing agent. It oxidizes ethanol to acetaldehyde.

The IUPAC name of acetaldehyde is ethanal. During oxidation of ethanol to ethanal, a molecule of hydrogen is removed and a carbon-oxygen single bond is converted to a carbon-oxygen double bond. During this reaction, the hydroxyl group of alcohol is converted to the carbonyl group of aldehyde.
The oxidation of ethanol to ethanol in presence of acidified potassium dichromate does not stop at the aldehyde stage. In the further oxidation, ethanal is converted to ethanoic acid.
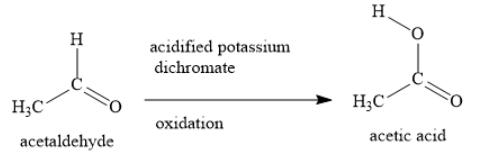
You can also call ethanoic acid as acetic acid. It is a carboxylic acid.
Hence, ethanol on reaction of acidified potassium dichromate form ethanoic acid. Hence, the correct option is the option D.
Note:
During the reaction, potassium dichromate (orange colour) is reduced to chromium(III) ions (green colour).
If you want the oxidation to stop at the aldehyde stage then you need to use pyridinium chlorochromate (PCC) or pyridinium dichromate (PDC).
Complete answer:
The structure of ethanol is as shown below:

Ethanol is an alcohol. It is also called ethyl alcohol. It contains two carbon atoms as part of an ethyl group and one hydroxyl group.
Acidified potassium dichromate is a strong oxidizing agent. It oxidizes ethanol to acetaldehyde.

The IUPAC name of acetaldehyde is ethanal. During oxidation of ethanol to ethanal, a molecule of hydrogen is removed and a carbon-oxygen single bond is converted to a carbon-oxygen double bond. During this reaction, the hydroxyl group of alcohol is converted to the carbonyl group of aldehyde.
The oxidation of ethanol to ethanol in presence of acidified potassium dichromate does not stop at the aldehyde stage. In the further oxidation, ethanal is converted to ethanoic acid.

You can also call ethanoic acid as acetic acid. It is a carboxylic acid.
Hence, ethanol on reaction of acidified potassium dichromate form ethanoic acid. Hence, the correct option is the option D.
Note:
During the reaction, potassium dichromate (orange colour) is reduced to chromium(III) ions (green colour).
If you want the oxidation to stop at the aldehyde stage then you need to use pyridinium chlorochromate (PCC) or pyridinium dichromate (PDC).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




