
Ethanoic acid is the IUPAC name of acetic acid.
a.) True
b.) False
Answer
601.8k+ views
Hint: Start this question by breaking up the name of ethanoic acid. As the name suggests, ethanoic acid is made from ‘eth-’ and ‘-oic acid’. It is one of the simplest carboxylic acids. It is made from some ‘acetic acid bacteria’.
Complete step by step answer:
Let us try to draw the structure of ethanoic acids by breaking up the name of the compound. It is made up of -
‘eth-’ which indicates that the compound is made up of two carbons.
‘-an-’ which indicates that the compound is an alkane.
‘-oic acid’ which indicates that the compound is a carboxylic acid.
Now, let us draw the structure of the compound.
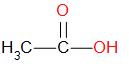 Ethanoic acid is commonly known as acetic acid, which is derived from the Latin word ‘acetum’.
Ethanoic acid is commonly known as acetic acid, which is derived from the Latin word ‘acetum’.
It is a weak acid, because it does not dissociate completely in aqueous solution. Acetic acid dissociates as –
\[C{{H}_{3}}COOH\rightleftharpoons C{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\]
It is a monoprotic acid (releases one proton in aqueous solution).
Therefore, the answer is – option (a) – The given statement is true.
Additional Information:
Vinegar is 4% acetic acid by volume. It has a pKa of around 4.7. It has a very distinctive sour taste and pungent smell.
Note: Acetic acid is generally produced from bacterial fermentation, more specifically - Acetobacter. The substrate can be fermented grain, malt, rice or potato masher. It is made by carbonylation of methanol (introduction of CO in methanol). It is also produced from oxidation of acetaldehyde.
Complete step by step answer:
Let us try to draw the structure of ethanoic acids by breaking up the name of the compound. It is made up of -
‘eth-’ which indicates that the compound is made up of two carbons.
‘-an-’ which indicates that the compound is an alkane.
‘-oic acid’ which indicates that the compound is a carboxylic acid.
Now, let us draw the structure of the compound.

It is a weak acid, because it does not dissociate completely in aqueous solution. Acetic acid dissociates as –
\[C{{H}_{3}}COOH\rightleftharpoons C{{H}_{3}}CO{{O}^{-}}+{{H}^{+}}\]
It is a monoprotic acid (releases one proton in aqueous solution).
Therefore, the answer is – option (a) – The given statement is true.
Additional Information:
Vinegar is 4% acetic acid by volume. It has a pKa of around 4.7. It has a very distinctive sour taste and pungent smell.
Note: Acetic acid is generally produced from bacterial fermentation, more specifically - Acetobacter. The substrate can be fermented grain, malt, rice or potato masher. It is made by carbonylation of methanol (introduction of CO in methanol). It is also produced from oxidation of acetaldehyde.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




