
When esters are refluxed with metallic sodium in an aprotic solvent like ether, \[\alpha \]-hydroxy ketones are formed. This reaction is known as:
A.Acyloin condensation
B.Aldol condensation
C.Cross Aldol condensation.
D.Self aldol condensation.
Answer
574.2k+ views
Hint: Since the reagents mentioned in the question are metallic sodium in ether (an aprotic solvent), it is clear that it is not the ideal reagent for aldol condensation to happen. So, we need to know the naming reactions thoroughly.
Complete step by step answer:
To get a better insight, we will be listing the detailed review on the options
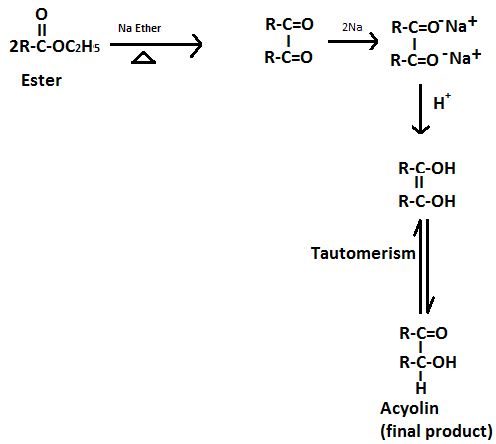
Option A. Acyloin condensation-
Reagent in this reaction: sodium in ether
Option B. Aldol condensation:
Reagent in this reaction: diluted bases giving \[O{H^ - }\]
In actual it is of three types:
Self aldol (option C.) and cross aldol (option D.) condensation.Intermolecular aldol condensation also occurs in which five membered or six membered rings are formed.
Carbonyl compounds containing at least one acidic $alpha$ hydrogen react with dilute solution of bases (containing an \[O{H^ - }\] group) to form unsaturated aldehydes. Such a process is called aldol condensation.
Reaction follows as:
\[
HCHO + C{H_3}CHO\xrightarrow{{O{H^ - }diluted}}{H_2}C(OH)C{H_2}CHO \\
\\
\]
This is an example of cross aldol condensation, where reactants are Methanal and Ethanal and the product formed is \[3 - hydroxypropanal\].
Thus, option A. Acyloin condensation is the correct solution to the given question.
Note:
For aldol condensation, \[\alpha \]-hydrogen is a must in the carbonyl compound. This reaction occurs in the presence of dilute solutions of sodium hydroxide, or potassium hydroxide. Bases like barium hydroxide and calcium hydroxide can also support aldol condensation.
Complete step by step answer:
To get a better insight, we will be listing the detailed review on the options
Option A. Acyloin condensation-
Reagent in this reaction: sodium in ether

Option B. Aldol condensation:
Reagent in this reaction: diluted bases giving \[O{H^ - }\]
In actual it is of three types:
Self aldol (option C.) and cross aldol (option D.) condensation.Intermolecular aldol condensation also occurs in which five membered or six membered rings are formed.
Carbonyl compounds containing at least one acidic $alpha$ hydrogen react with dilute solution of bases (containing an \[O{H^ - }\] group) to form unsaturated aldehydes. Such a process is called aldol condensation.
Reaction follows as:
\[
HCHO + C{H_3}CHO\xrightarrow{{O{H^ - }diluted}}{H_2}C(OH)C{H_2}CHO \\
\\
\]
This is an example of cross aldol condensation, where reactants are Methanal and Ethanal and the product formed is \[3 - hydroxypropanal\].
Thus, option A. Acyloin condensation is the correct solution to the given question.
Note:
For aldol condensation, \[\alpha \]-hydrogen is a must in the carbonyl compound. This reaction occurs in the presence of dilute solutions of sodium hydroxide, or potassium hydroxide. Bases like barium hydroxide and calcium hydroxide can also support aldol condensation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




