
What is the empirical formula of melamine?
Answer
516.3k+ views
Hint: We know that the empirical formula provides the idea about the lowest whole number ratio in which atoms are exiting in the compound. It does not give any idea about the arrangement of the atoms in the compound. It is the relative ratio of elements in a compound.
Complete answer:
The empirical formula is the simplest integer ratio in which atoms combine. The relative number of atoms in the compound is explained by considering the empirical formula. The Empirical formula can be calculated by following steps:
- Start with the number of grams of each element of the compound given in the problem (If percentages are given, assume that the total mass is grams so that the mass of each element will be equal to the percentage given)
- Convert the mass of each element into moles using the molar mass of the respective elements from the periodic table.
- Divide the value of each mole by the smallest number of moles calculated.
- Round to the nearest whole number. This is the mole ratio of the elements and is represented by subscripts in the empirical formula.
- If the number is too far to round up, then multiply each solution by the same factor to get the lowest possible whole number multiple.
1.
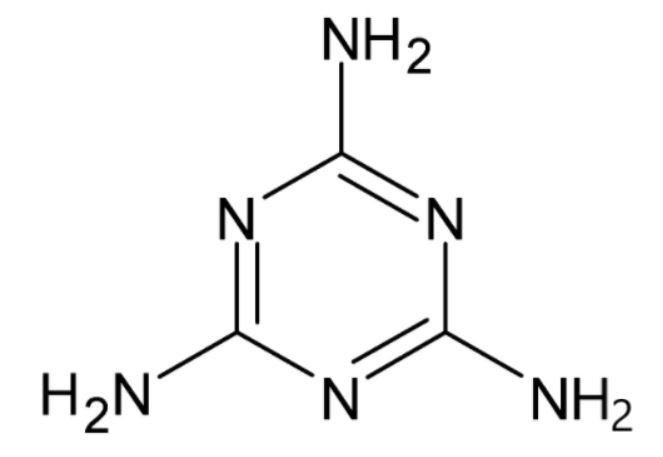
The molecular formula is $ {{C}_{3}}{{H}_{6}}{{N}_{6}}. $ The empirical formula tells us the simplest whole-number ratio of the different types of atoms in a compound. To get the empirical formula, we divide the subscripts in the molecular formula by their highest common factor $ \left( 3 \right). $ This gives you the empirical formula $ C{{H}_{2}}{{N}_{2}}. $
Note:
Remember that don't get confused between molecular formula and empirical formula. Empirical formulas show the simplest whole-number ratio of atoms in a compound whereas molecular formulas show the number of each type of atom in a molecule.
Complete answer:
The empirical formula is the simplest integer ratio in which atoms combine. The relative number of atoms in the compound is explained by considering the empirical formula. The Empirical formula can be calculated by following steps:
- Start with the number of grams of each element of the compound given in the problem (If percentages are given, assume that the total mass is grams so that the mass of each element will be equal to the percentage given)
- Convert the mass of each element into moles using the molar mass of the respective elements from the periodic table.
- Divide the value of each mole by the smallest number of moles calculated.
- Round to the nearest whole number. This is the mole ratio of the elements and is represented by subscripts in the empirical formula.
- If the number is too far to round up, then multiply each solution by the same factor to get the lowest possible whole number multiple.
1.

The molecular formula is $ {{C}_{3}}{{H}_{6}}{{N}_{6}}. $ The empirical formula tells us the simplest whole-number ratio of the different types of atoms in a compound. To get the empirical formula, we divide the subscripts in the molecular formula by their highest common factor $ \left( 3 \right). $ This gives you the empirical formula $ C{{H}_{2}}{{N}_{2}}. $
Note:
Remember that don't get confused between molecular formula and empirical formula. Empirical formulas show the simplest whole-number ratio of atoms in a compound whereas molecular formulas show the number of each type of atom in a molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




