
During the development of an exposed camera film, one step is involves the following reaction:
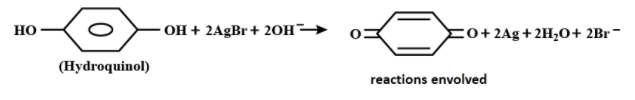
Which of the following best describes the role of hydroquinol
(A) It acts as an acid
(B) It acts as a reducing agent
(C) It acts as oxidant
(D) It acts as a base

Answer
573.9k+ views
Hint: Photographic film is basically a strip of transparent plastic that is covered in or coated with a gelatin-like substance known as emulsion. This emulsion contains microscopically small silver halides crystals. These silver halide crystals are sensitive to light.
Complete step by step solution:
Before we move forward to the solution of the question let's first understand some basic concepts. If exposed directly to light this emulsion would darken and the film would then become useless. To counter this issue the exposure time of the film is so adjusted that only a very slight chemical change which is proportional to the amount of light which is absorbed by each crystal occurs. This results in the formation of the latent image in the emulsion. This is used to develop a visible image.
In the above equation mentioned silver ion gains one electron to form silver, the equation involved is shown below:
\[A{{g}^{+}}+e\to Ag\]
In the left hand side of the reaction silver is present in +1 oxidation state and in the right hand side of the reaction silver is present in 0 oxidation state hence hydroquinol acts as a reducing agent.
Hence, the correct answer is option (B) i.e. the role of hydroquinol is that it acts as a reducing agent.
Note: These films are sensitive to the ultraviolet light, the gamma rays and the X rays and the particles having high energy as well. Silver is precipitated to form the actual visible image on the developed image.
Complete step by step solution:
Before we move forward to the solution of the question let's first understand some basic concepts. If exposed directly to light this emulsion would darken and the film would then become useless. To counter this issue the exposure time of the film is so adjusted that only a very slight chemical change which is proportional to the amount of light which is absorbed by each crystal occurs. This results in the formation of the latent image in the emulsion. This is used to develop a visible image.
In the above equation mentioned silver ion gains one electron to form silver, the equation involved is shown below:
\[A{{g}^{+}}+e\to Ag\]
In the left hand side of the reaction silver is present in +1 oxidation state and in the right hand side of the reaction silver is present in 0 oxidation state hence hydroquinol acts as a reducing agent.
Hence, the correct answer is option (B) i.e. the role of hydroquinol is that it acts as a reducing agent.
Note: These films are sensitive to the ultraviolet light, the gamma rays and the X rays and the particles having high energy as well. Silver is precipitated to form the actual visible image on the developed image.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




