
Draw the structures of the following molecules:
(i) \[Xe{{F}_{2}}\]
(ii) \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]
Answer
554.1k+ views
Hint: Xenon is an inert gas, so it has a completely filled electronic configuration. While making bonds with another atom or ion, it usually makes space for the incoming electrons, by the transition of outermost electrons to the empty d orbital.
In case of Peroxydisulfuric Acid, the structure contains a peroxy bond as in the two oxygen at the middle are attached to one another through a single bond.
Complete step by step answer:
The ground state electronic configuration of the central atom xenon is \[\left[ Kr \right]\text{ }4{{d}^{10}}\text{ }5s{}^\text{2}\text{ }5{{p}^{6}}\]
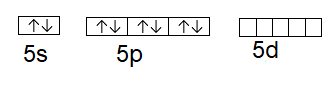
The orbitals can be represented as above, here we can see that the \[s\]and \[p\]orbitals are fully filled as in case of xenon, and the d orbital is empty.
Now we will write the excited state electronic configuration of the xenon, so that it becomes suitable for the incoming fluorine, and make room for its electrons.
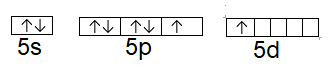
Now we can see in the excited state, one electron from the \[p\] orbital jumps to the empty \[d\] orbital, now the incoming fluorine will give its electrons in those two orbitals.
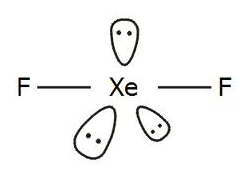
As we can see the central atom contains three lone pairs and two of the sites in the orbital which had rooms for the electrons of fluorine are now adjusted.
On the other hand, if we see the structure of \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\], Peroxydisulfuric Acid contains a peroxy bond, as in the two oxygen at the middle are attached to one another through a single bond. It is a colourless solid and one of the most useful peroxyacid oxidants which are widely available. Peroxydisulfuric Acid is an inorganic compound with the chemical formula \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]. It can be prepared from ammonium or potassium persulfate in an acidic solution. It is an anhydride of sulphuric and peroxydisulfuric acid and may be prepared by oxidation of oleum with ozone or hydrogen peroxide.
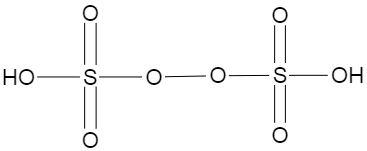
Note: Peroxysulphuricacid is also called Marshall’s acid, and can be produced by electrolyzing an aqueous solution which contains sulphate ions in an electrolysis reactor should be produced by electrolyzing.
Xenon difluoride has an application of a powerful fluorinating agent with the chemical formula \[Xe{{F}_{2}}\] , and it is one of the most stable xenon compounds known to us.
In case of Peroxydisulfuric Acid, the structure contains a peroxy bond as in the two oxygen at the middle are attached to one another through a single bond.
Complete step by step answer:
The ground state electronic configuration of the central atom xenon is \[\left[ Kr \right]\text{ }4{{d}^{10}}\text{ }5s{}^\text{2}\text{ }5{{p}^{6}}\]

The orbitals can be represented as above, here we can see that the \[s\]and \[p\]orbitals are fully filled as in case of xenon, and the d orbital is empty.
Now we will write the excited state electronic configuration of the xenon, so that it becomes suitable for the incoming fluorine, and make room for its electrons.

Now we can see in the excited state, one electron from the \[p\] orbital jumps to the empty \[d\] orbital, now the incoming fluorine will give its electrons in those two orbitals.
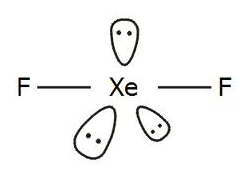
As we can see the central atom contains three lone pairs and two of the sites in the orbital which had rooms for the electrons of fluorine are now adjusted.
On the other hand, if we see the structure of \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\], Peroxydisulfuric Acid contains a peroxy bond, as in the two oxygen at the middle are attached to one another through a single bond. It is a colourless solid and one of the most useful peroxyacid oxidants which are widely available. Peroxydisulfuric Acid is an inorganic compound with the chemical formula \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]. It can be prepared from ammonium or potassium persulfate in an acidic solution. It is an anhydride of sulphuric and peroxydisulfuric acid and may be prepared by oxidation of oleum with ozone or hydrogen peroxide.

Note: Peroxysulphuricacid is also called Marshall’s acid, and can be produced by electrolyzing an aqueous solution which contains sulphate ions in an electrolysis reactor should be produced by electrolyzing.
Xenon difluoride has an application of a powerful fluorinating agent with the chemical formula \[Xe{{F}_{2}}\] , and it is one of the most stable xenon compounds known to us.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




