
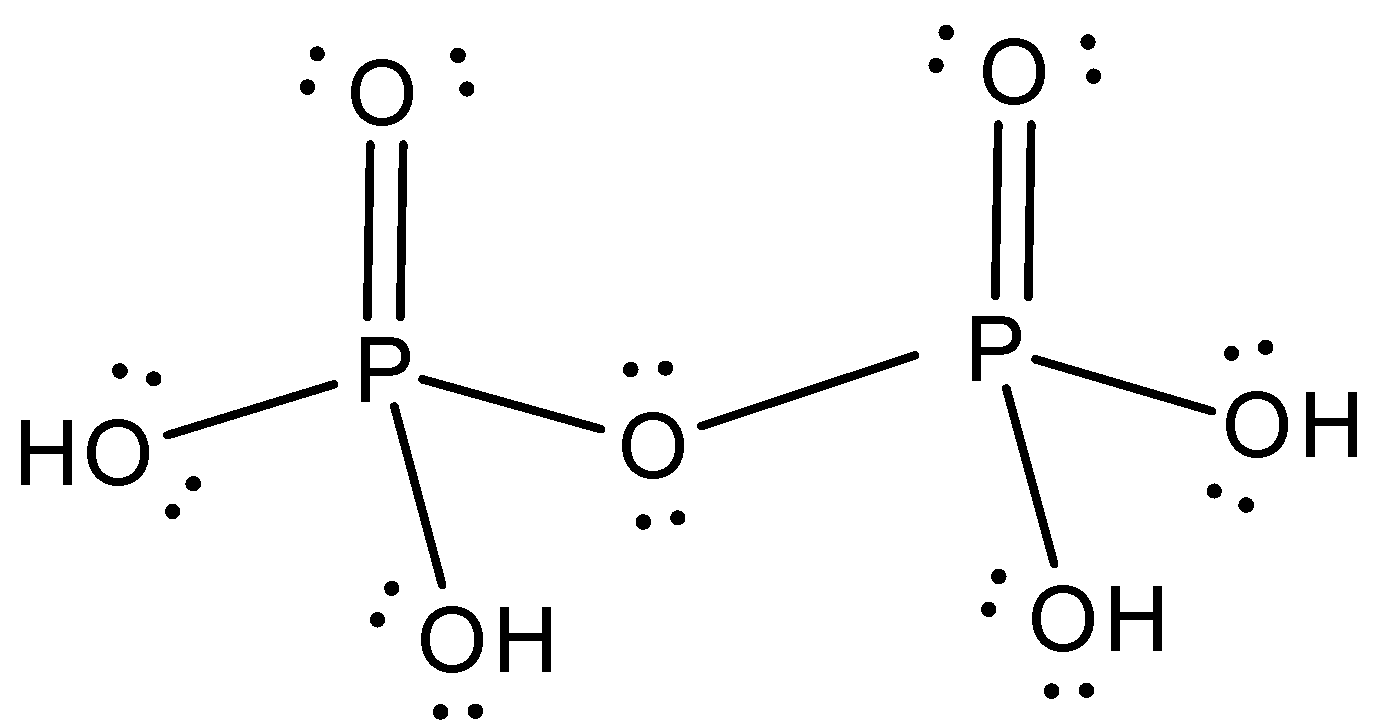
Draw the electron dot formula of ${H_4}{P_2}{O_7}$
Answer
569.4k+ views
Hint: We as a whole realize Electron dot structure:
A Lewis dot chart could likewise be a portrayal of the valence electrons of a particle that utilizes dots around the symbol of the component. The amount of spots approaches the amount of valence electrons inside the particle.
Complete step by step answer:
We realize that the Lewis structure is that the basic portrayal of the measure of valence electrons during an atom. To draw the Lewis structures of a particle initially ascertain the whole number of valence electrons inside the molecule.
There are valence electrons for the Lewis structure. Carbon is that the littlest sum electronegative particle and goes inside the center of this structure. The Lewis structure requires you've twofold connections between the carbons and sulfur molecules so as to fill the octet of carbon.
The Lewis structure of ${H_4}{P_2}{O_7}$ is,
Note: We can characterize the move of electrons as a cycle during which an electron shares at least one electron to its neighboring atoms. We as a whole realize that there must be eight electrons inside the peripheral orbital of a particle. This is regularly alluded to as an octet rule. On the off chance that a particle has yet eight electrons, they tend to respond and yield stable compounds.
We can state octet rule, as "An atom is steadier when their peripheral shells are packed with eight electrons". Particles like incandescent lamp, oxygen, nitrogen and carbon comply with the octet rule. All the climate of the most gathering complies with the octet rule.
We know that there are two main sorts of bonds. They are,
1) Ionic bonds
2) Covalent bonds
Ionic bonds are shaped by gratitude to the exchange of electrons from one molecule to another. This by and large occurs in metal. Ionic mixes such normal salt, potassium chloride have ionic bonds in the middle of their atoms.
A Lewis dot chart could likewise be a portrayal of the valence electrons of a particle that utilizes dots around the symbol of the component. The amount of spots approaches the amount of valence electrons inside the particle.
Complete step by step answer:
We realize that the Lewis structure is that the basic portrayal of the measure of valence electrons during an atom. To draw the Lewis structures of a particle initially ascertain the whole number of valence electrons inside the molecule.
There are valence electrons for the Lewis structure. Carbon is that the littlest sum electronegative particle and goes inside the center of this structure. The Lewis structure requires you've twofold connections between the carbons and sulfur molecules so as to fill the octet of carbon.
The Lewis structure of ${H_4}{P_2}{O_7}$ is,

Note: We can characterize the move of electrons as a cycle during which an electron shares at least one electron to its neighboring atoms. We as a whole realize that there must be eight electrons inside the peripheral orbital of a particle. This is regularly alluded to as an octet rule. On the off chance that a particle has yet eight electrons, they tend to respond and yield stable compounds.
We can state octet rule, as "An atom is steadier when their peripheral shells are packed with eight electrons". Particles like incandescent lamp, oxygen, nitrogen and carbon comply with the octet rule. All the climate of the most gathering complies with the octet rule.
We know that there are two main sorts of bonds. They are,
1) Ionic bonds
2) Covalent bonds
Ionic bonds are shaped by gratitude to the exchange of electrons from one molecule to another. This by and large occurs in metal. Ionic mixes such normal salt, potassium chloride have ionic bonds in the middle of their atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




