
Draw the branched structural formula of:
(A) Methoxy methane.
(B) $ 2- $ methylpropan $ -2- $ ol.
Answer
520.8k+ views
Hint :We know that for this problem, we should know about the general points of the IUPAC naming while naming the compound like the naming of functional groups, alkyl groups, chains, etc. So that we can easily draw the structure of the molecule by using its name.
Complete Step By Step Answer:
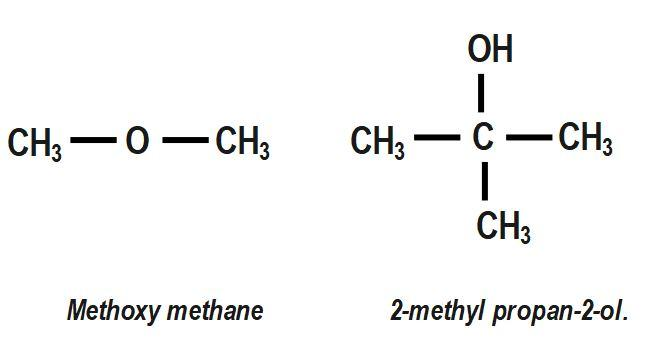
In the given question we have to draw the structural formula of the given compound that is a $ 2- $ methylpropan $ -2- $ ol and Methoxy methane molecule. IUPAC or International Union of Pure and Applied Compounds are a method of naming any organic compound. There are some points to be remembered while names such as: If there is one carbon atom in the longest chain then it is named as meth, if two carbon atoms are present then it is named as eth and if three carbon atoms are present then it is named as a prop. So, as we can see that the main chain is named as 'prop' so it consists of three carbon atoms with one methyl group that is one carbon atom at $ 2- $ carbon.
Now, the word 'ane' means that there is a single bond present only because for double bond 'ene' is used and for a triple bond, we use 'yne'. Now, the last one is the functional group 'ol' which is a suffix for an alcohol group. So, both the structural formula of the given compound will be:
Note :
Remember that for other functional groups such as carboxylic acid the suffix -oic acid is used, for aldehyde -al is used, etc. The naming of the functional group is also dependent on the priority order of the functional groups.
Complete Step By Step Answer:
In the given question we have to draw the structural formula of the given compound that is a $ 2- $ methylpropan $ -2- $ ol and Methoxy methane molecule. IUPAC or International Union of Pure and Applied Compounds are a method of naming any organic compound. There are some points to be remembered while names such as: If there is one carbon atom in the longest chain then it is named as meth, if two carbon atoms are present then it is named as eth and if three carbon atoms are present then it is named as a prop. So, as we can see that the main chain is named as 'prop' so it consists of three carbon atoms with one methyl group that is one carbon atom at $ 2- $ carbon.
Now, the word 'ane' means that there is a single bond present only because for double bond 'ene' is used and for a triple bond, we use 'yne'. Now, the last one is the functional group 'ol' which is a suffix for an alcohol group. So, both the structural formula of the given compound will be:

Note :
Remember that for other functional groups such as carboxylic acid the suffix -oic acid is used, for aldehyde -al is used, etc. The naming of the functional group is also dependent on the priority order of the functional groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




