
Draw Newman and Sawhorse projections for the eclipsed and staggered conformations of ethane. Which of these conformations is more stable and why?
Answer
517.8k+ views
Hint: We have to know that, in an obscured adaptation the carbons are adjusted so the hydrogens are agreed with one another. This makes a steric block between them. In a stunned conformity, the atoms are for the most part similarly, dispersed from one another. The overshadowed conformity of ethane is less steady than the stunned adaptation.
Complete answer:
We have to know that sawhorse Projections can likewise be drawn so the gatherings on the front carbon are amazed or overshadowed with the gatherings on the back carbon. The following are two Sawhorse Projections of ethane. The design on the left is stunned, and the construction on the privilege is overshadowed.
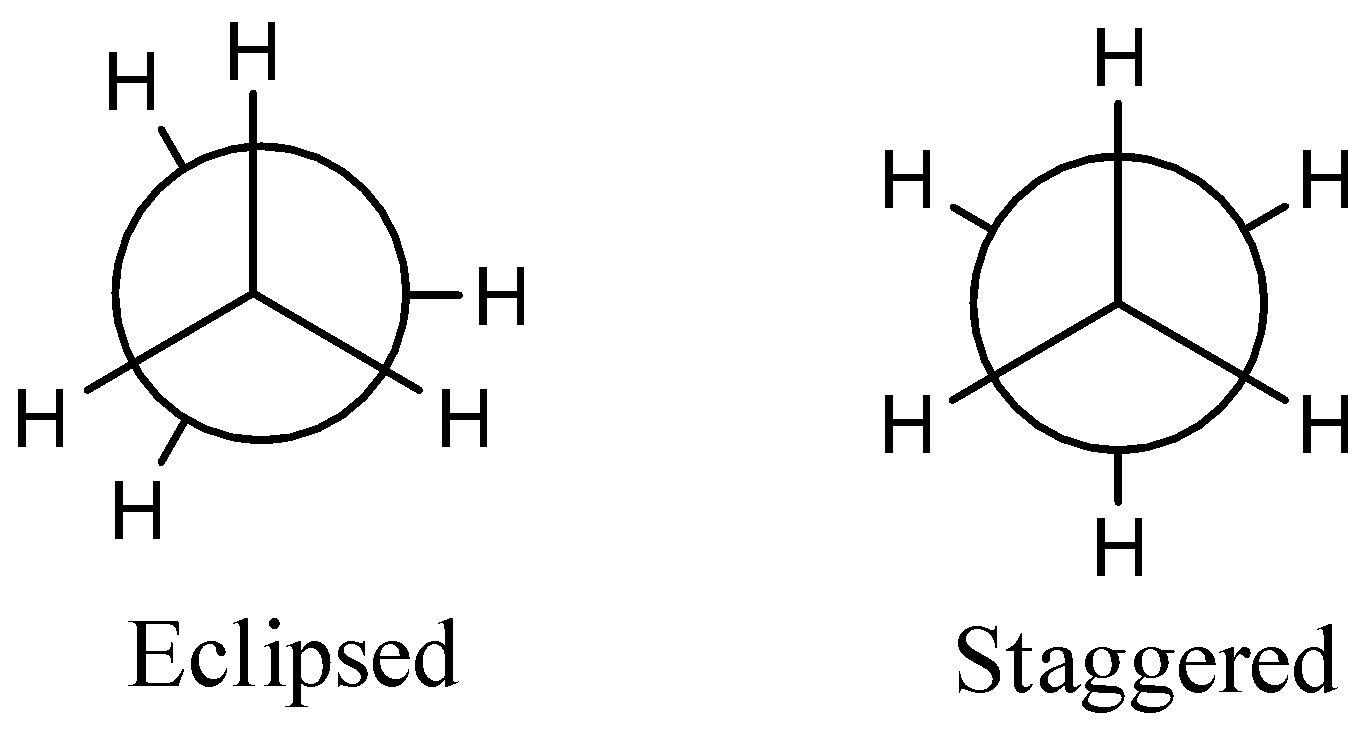
We have to see, in a Newman projection, the carbon-carbon bond is seen along its pivot and the two carbon particles are addressed by a circle. The bonds appended to the front carbon are addressed by lines going to the focal point of the circle, and bonds joined to the back carbon are addressed by lines going to the edge of the circle.
The structure of Newman projection,
The structure of sawhorse projection,
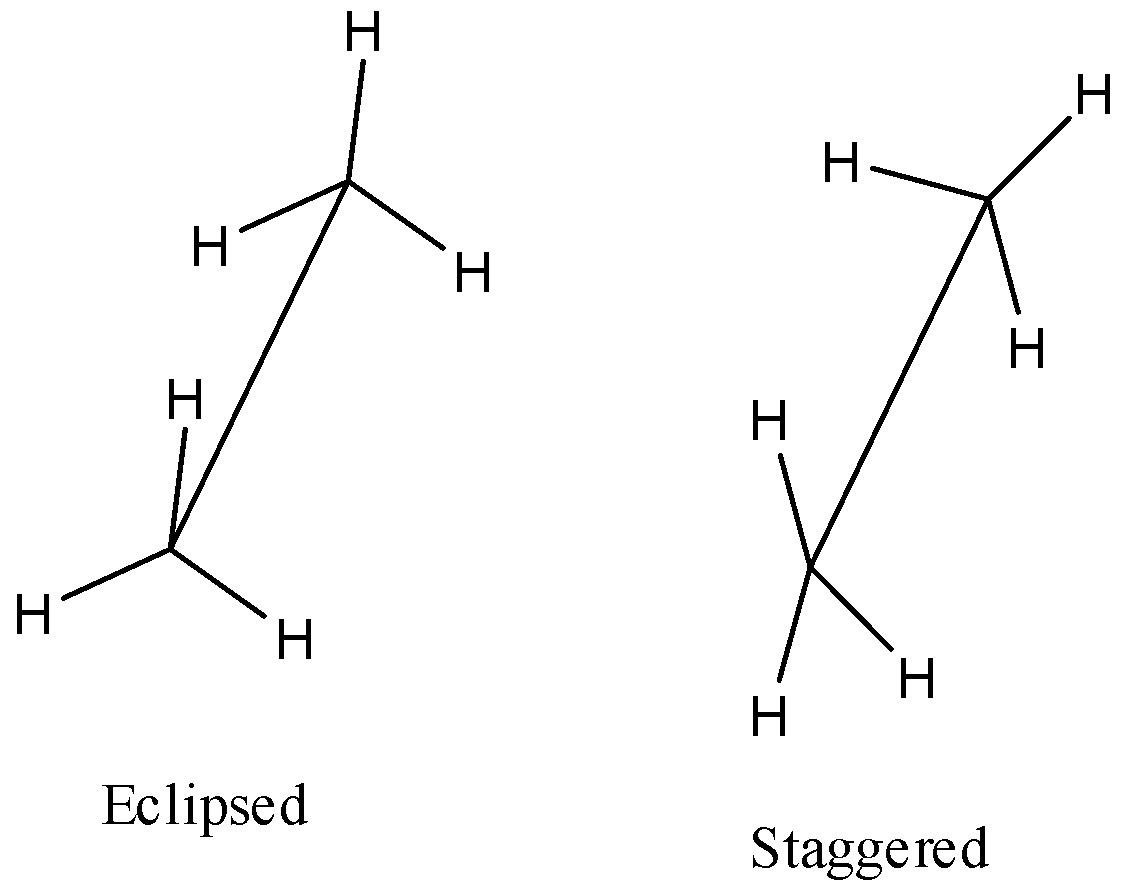
We have to see, in the staggered type of ethane, the electron billows of carbon-hydrogen bonds are as far separated as could be expected. Hence, there are least loathsome powers, least energy, and most extreme solidness of the atom. Then again, when the amazing structure changes into the eclipsed structure, the electron billows of the carbon-hydrogen bonds come nearer to one another subsequent in an expansion in electron cloud aversions. To check the expanded horrible powers, the atom should have more energy and consequently has lesser solidness.
Note:
We have to know that these eclipses and staggers give the construction of an atom when glanced through the carbon spine from the front-back bearing. The primary distinction between staggered adaptation and eclipsed compliance is that amazed conformity has lower possible energy while obscured conformity has the most extreme expected energy.
Complete answer:
We have to know that sawhorse Projections can likewise be drawn so the gatherings on the front carbon are amazed or overshadowed with the gatherings on the back carbon. The following are two Sawhorse Projections of ethane. The design on the left is stunned, and the construction on the privilege is overshadowed.
We have to see, in a Newman projection, the carbon-carbon bond is seen along its pivot and the two carbon particles are addressed by a circle. The bonds appended to the front carbon are addressed by lines going to the focal point of the circle, and bonds joined to the back carbon are addressed by lines going to the edge of the circle.
The structure of Newman projection,

The structure of sawhorse projection,

We have to see, in the staggered type of ethane, the electron billows of carbon-hydrogen bonds are as far separated as could be expected. Hence, there are least loathsome powers, least energy, and most extreme solidness of the atom. Then again, when the amazing structure changes into the eclipsed structure, the electron billows of the carbon-hydrogen bonds come nearer to one another subsequent in an expansion in electron cloud aversions. To check the expanded horrible powers, the atom should have more energy and consequently has lesser solidness.
Note:
We have to know that these eclipses and staggers give the construction of an atom when glanced through the carbon spine from the front-back bearing. The primary distinction between staggered adaptation and eclipsed compliance is that amazed conformity has lower possible energy while obscured conformity has the most extreme expected energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




