
Draw labelled diagram of Laboratory method of phosphine and give chemical equation?
Answer
584.1k+ views
Hint: Phosphine is one of the most common hydrides of phosphorus. It is colourless and flammable in nature. It is also slightly soluble in water.
Complete step by step answer:
The production of phosphine can be carried out using the following process:
The reactants to be used are white phosphorus and sodium hydroxide. Both these reactants are allowed to react in an inert atmosphere. This inert atmosphere mainly consists of carbon dioxide gas. The chemical reaction for this process can be given as:
\[{P_4} + 3NaOH + {H_2}O \to P{H_3} + 3Na{H_2}P{O_2}\]
Also, the experimental - setup for the production of phosphine can be shown as:
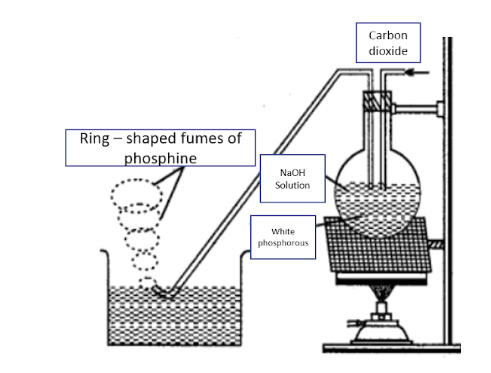
Additional Information:
The products that are formed on the other side phosphine and sodium hypophosphite. Now the phosphine formed might be a little impure. These impurities may include \[{P_2}{H_4}\]or \[{P_4}\]. These impurities are present in the vapour form. They can be removed by absorbing it in hydrogen iodide in order to form phosphonium iodide, which upon further treatment with potassium iodide gives off pure phosphine.
Note:
White phosphorous is one of the forms in which elemental phosphorus exists, because phosphorous cannot be found as a freely existing element. It also emits a kind of a faint glow when exposed to oxygen. The other form of phosphorus, red phosphorus does not show such a luminescence when reacting with oxygen.
Complete step by step answer:
The production of phosphine can be carried out using the following process:
The reactants to be used are white phosphorus and sodium hydroxide. Both these reactants are allowed to react in an inert atmosphere. This inert atmosphere mainly consists of carbon dioxide gas. The chemical reaction for this process can be given as:
\[{P_4} + 3NaOH + {H_2}O \to P{H_3} + 3Na{H_2}P{O_2}\]
Also, the experimental - setup for the production of phosphine can be shown as:

Additional Information:
The products that are formed on the other side phosphine and sodium hypophosphite. Now the phosphine formed might be a little impure. These impurities may include \[{P_2}{H_4}\]or \[{P_4}\]. These impurities are present in the vapour form. They can be removed by absorbing it in hydrogen iodide in order to form phosphonium iodide, which upon further treatment with potassium iodide gives off pure phosphine.
Note:
White phosphorous is one of the forms in which elemental phosphorus exists, because phosphorous cannot be found as a freely existing element. It also emits a kind of a faint glow when exposed to oxygen. The other form of phosphorus, red phosphorus does not show such a luminescence when reacting with oxygen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




