
how can I draw fischer projections from wedge and dash?
Answer
548.4k+ views
Hint: Using wedge and dash notation, solid lines (sticks) represent chemical bonds in the plane of the surface.
Black wedges represent chemical bonds coming toward you, while dashed lines are for bonds that extend back behind the surface
.We must view a wedge-dash formula from the correct angle to convert it to a Fischer projection.
Complete step by step answer:
Here's the wedge-dash structure.
EG GLUCOSE:
We now view the molecule with \[\text{C}-1\] at the top and with all chiral carbons closest to our eye.
If we are viewing from above, we must mentally rotate the bonds so that \[\text{C}-2\] and \[\text{C}-4\]
are pointing "up".
When we do this, the wedges become dashes, and the dashes become wedges, as in the picture below.
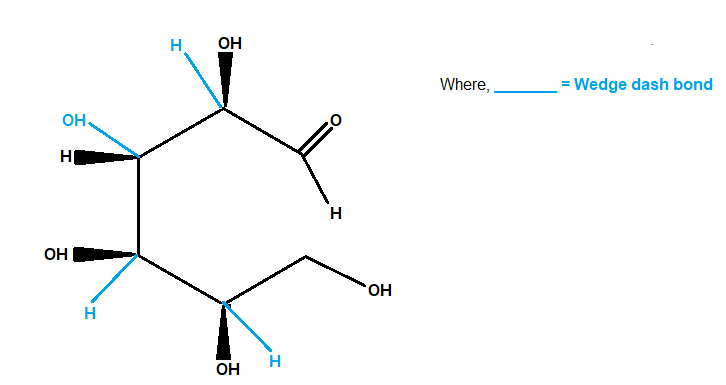
Glucose
So the \[\text{OH}\] groups on \[\text{C}-2\] and \[\text{C}-4\] become wedges.
We don't rotate \[\text{C}-3\] and\[\text{C}-5\] , so the bonds to the \[\text{OH}\]groups on those atoms remain the same.
Note: The wedge-dash formula now looks like the one in the image below (I cropped it from here).
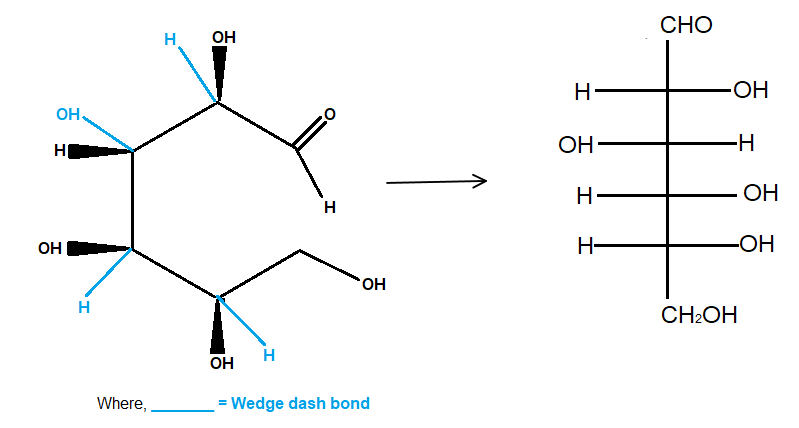 Fischer
Fischer
The wedges are now on the right, and the dashes are on the left.
It is as if we had wrapped the chain around a cylindrical tube.
When you flatten the structure onto the surface of the cylinder, you get the Fischer projection of D-glucose.
understanding the concept of wedge and dash helps to understand stereochemistry and spectroscopy which is an important aspect in research
Black wedges represent chemical bonds coming toward you, while dashed lines are for bonds that extend back behind the surface
.We must view a wedge-dash formula from the correct angle to convert it to a Fischer projection.
Complete step by step answer:
Here's the wedge-dash structure.

EG GLUCOSE:
We now view the molecule with \[\text{C}-1\] at the top and with all chiral carbons closest to our eye.
If we are viewing from above, we must mentally rotate the bonds so that \[\text{C}-2\] and \[\text{C}-4\]
are pointing "up".
When we do this, the wedges become dashes, and the dashes become wedges, as in the picture below.

Glucose
So the \[\text{OH}\] groups on \[\text{C}-2\] and \[\text{C}-4\] become wedges.
We don't rotate \[\text{C}-3\] and\[\text{C}-5\] , so the bonds to the \[\text{OH}\]groups on those atoms remain the same.
Note: The wedge-dash formula now looks like the one in the image below (I cropped it from here).

The wedges are now on the right, and the dashes are on the left.
It is as if we had wrapped the chain around a cylindrical tube.
When you flatten the structure onto the surface of the cylinder, you get the Fischer projection of D-glucose.
understanding the concept of wedge and dash helps to understand stereochemistry and spectroscopy which is an important aspect in research
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




