
Draw each of the possible stereoisomers of the octahedral complexes listed: $ (a)Ma3bcd,(b)Ma2bcde~ $ and $ (c)M(AA)(AA)cd.\text{ } $ the lower-case letters a, b, c d and e represent monodentate ligands, and upper-case letters (AA) represent the donor atoms of a bidentate ligand. Indicate which isomers are optically active (chiral).
Answer
544.8k+ views
Hint: Isomers are compounds with the same molecular formula but different chemical structure. This property of compounds exhibiting isomers is called isomerism. To show optical isomerism, the metal centre must be chiral.
Complete step by step solution:
Let us consider octahedral complexes. We shall represent the ligands by small case letters a,b,c etc. and the metal by M. Let the first example be $ Mabcdef, $ ,in which all the ligands are different. We shall write the trans pairs of ligands in brackets in alphabetical order. In each isomer there will be three trans pairs. Ligands that are not within the same bracket are cis to each other.
The geometrical isomers of Mabcdef areas follow.
Mabcdef
M(ab)(cd)(ef), M(ab)(ce)(df), M(ab)(cf)(de)
M(ac)(bd)(ef), M(ac)(be)(df), M(ac)(bf)(de)
M(ad)(bc)(ef), M(ad)(be)(cf), M(ad)(bf)(ce)
M(ae)(bc)(df), M(ae)(bd)(cf), M(ae)(bf)(cd)
M(af)(bc)(de), M(af)(bd)(ce), M(af)(be)(cd)
Maabcde
M(aa)(bc)(de),M(aa)(bd)(ce), M(aa)(be)(cd)
M(ab)(ac)(de), M(ab)(ad)(ce), M(ab)(ae)(cd)
M(ac)(ad)(be), M(ac)(ae)(bd)
M(ad)(ae)(bc)
Maaabcd
M(aa)(ab)(cd), M(aa)(ac)(bd), M(aa)(ad)(bc)
M(ab)(ac)(ad)
Maabbcd
M(aa)(bb)(cd), M(aa)(bc)(bd)
M(ab)(ab)(cd), M(ab)(ac)(bd), M(ab)(ad)(bc)
M(ac)(ad)(bb)
Maabbcc
M(aa)(bb)(cc), M(aa)(bc)(bc)
M(ab)(ab)(cc), M(ab)(ac)(bc)
M(ac)(ac)(bb)
Remember, the ligands within a bracket are trans to each other. In an octahedral complex there are three trans pairs of ligands. In enumerating the geometrical isomers note that alphabetical order has been maintained. Each succeeding trans pair is either equivalent to the preceding as in (ab)(ab) or alphabetically comes later as in (ab)(cd) or
(ac)(bd). This ensures that there is no duplication of isomers.
For practice use this technique to enumerate the geometrical isomers in square planar complexes. There of course, will be only two trans pairs of ligands in sq pl complexes.
We have considered examples of only monodentate ligands but this method can be applied to bidentate ligands as well if you keep it in mind that the two donor atoms of a bidentate ligand cannot be trans to each other.
Let us represent the donor atoms of a bidentate ligand by upper case letters. For example, ethylenediamine by AA. Let us enumerate the geometrical isomers of the complex M AAbcde.
M AAbcde
M(Ab)(Ac)(de), M(Ab)(Ad)(ce), M(Ab)(Ae)(cd)
M(Ac)(Ad)(be), M(Ac)(Ae)(bd)
M(Ad)(Ae)(bc)
The Structure for the possible stereoisomers of the octahedral complexes listed: $ (a)Ma3bcd,(b)Ma2bcde $ and $ (c)M(AA)(AA)cd $ are given by:
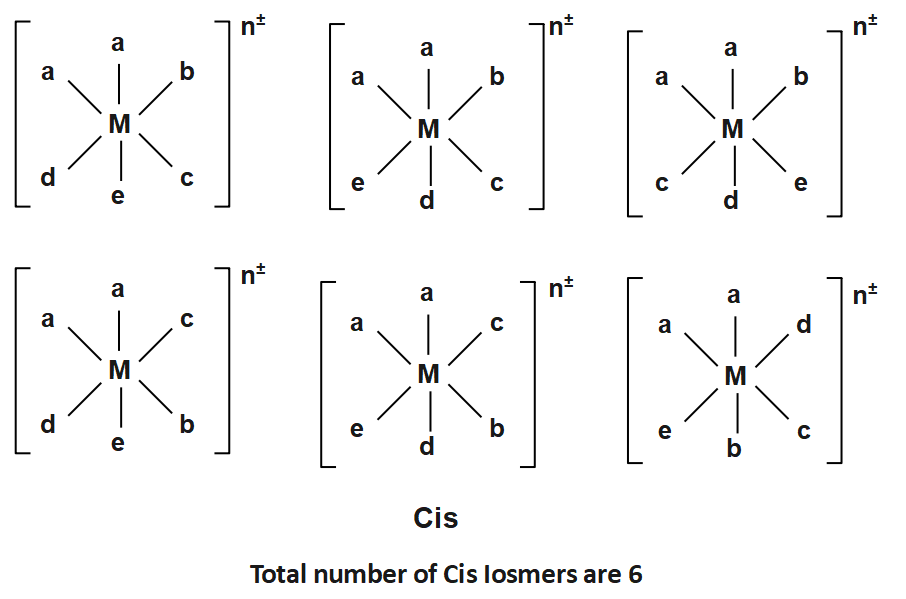
And
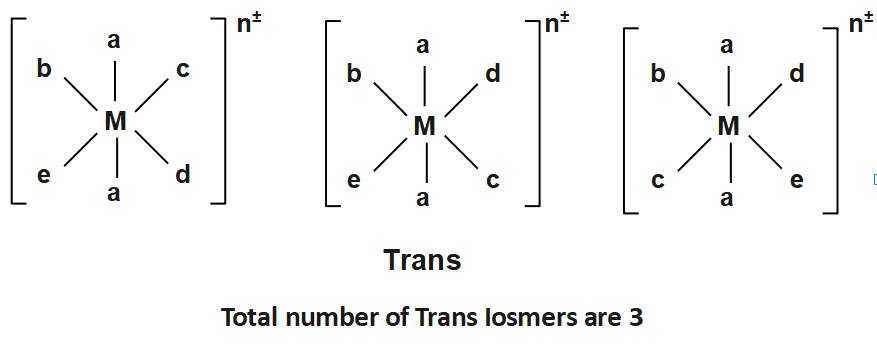
Note:
Note that the denticity can be defined as the number of donor atoms of a ligand which has the ability to attach to the central atom in a coordination complex. Ligands with one donor atom that have the ability to attach to the central atom are known as monodentate ligands whereas ligands with two donor atoms that have the ability to attach to the central atom are known as bidentate ligands.
Complete step by step solution:
Let us consider octahedral complexes. We shall represent the ligands by small case letters a,b,c etc. and the metal by M. Let the first example be $ Mabcdef, $ ,in which all the ligands are different. We shall write the trans pairs of ligands in brackets in alphabetical order. In each isomer there will be three trans pairs. Ligands that are not within the same bracket are cis to each other.
The geometrical isomers of Mabcdef areas follow.
Mabcdef
M(ab)(cd)(ef), M(ab)(ce)(df), M(ab)(cf)(de)
M(ac)(bd)(ef), M(ac)(be)(df), M(ac)(bf)(de)
M(ad)(bc)(ef), M(ad)(be)(cf), M(ad)(bf)(ce)
M(ae)(bc)(df), M(ae)(bd)(cf), M(ae)(bf)(cd)
M(af)(bc)(de), M(af)(bd)(ce), M(af)(be)(cd)
Maabcde
M(aa)(bc)(de),M(aa)(bd)(ce), M(aa)(be)(cd)
M(ab)(ac)(de), M(ab)(ad)(ce), M(ab)(ae)(cd)
M(ac)(ad)(be), M(ac)(ae)(bd)
M(ad)(ae)(bc)
Maaabcd
M(aa)(ab)(cd), M(aa)(ac)(bd), M(aa)(ad)(bc)
M(ab)(ac)(ad)
Maabbcd
M(aa)(bb)(cd), M(aa)(bc)(bd)
M(ab)(ab)(cd), M(ab)(ac)(bd), M(ab)(ad)(bc)
M(ac)(ad)(bb)
Maabbcc
M(aa)(bb)(cc), M(aa)(bc)(bc)
M(ab)(ab)(cc), M(ab)(ac)(bc)
M(ac)(ac)(bb)
Remember, the ligands within a bracket are trans to each other. In an octahedral complex there are three trans pairs of ligands. In enumerating the geometrical isomers note that alphabetical order has been maintained. Each succeeding trans pair is either equivalent to the preceding as in (ab)(ab) or alphabetically comes later as in (ab)(cd) or
(ac)(bd). This ensures that there is no duplication of isomers.
For practice use this technique to enumerate the geometrical isomers in square planar complexes. There of course, will be only two trans pairs of ligands in sq pl complexes.
We have considered examples of only monodentate ligands but this method can be applied to bidentate ligands as well if you keep it in mind that the two donor atoms of a bidentate ligand cannot be trans to each other.
Let us represent the donor atoms of a bidentate ligand by upper case letters. For example, ethylenediamine by AA. Let us enumerate the geometrical isomers of the complex M AAbcde.
M AAbcde
M(Ab)(Ac)(de), M(Ab)(Ad)(ce), M(Ab)(Ae)(cd)
M(Ac)(Ad)(be), M(Ac)(Ae)(bd)
M(Ad)(Ae)(bc)
The Structure for the possible stereoisomers of the octahedral complexes listed: $ (a)Ma3bcd,(b)Ma2bcde $ and $ (c)M(AA)(AA)cd $ are given by:

And

Note:
Note that the denticity can be defined as the number of donor atoms of a ligand which has the ability to attach to the central atom in a coordination complex. Ligands with one donor atom that have the ability to attach to the central atom are known as monodentate ligands whereas ligands with two donor atoms that have the ability to attach to the central atom are known as bidentate ligands.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




