
Draw all possible geometrical isomers of ${[Pt(IV){(N{H_3})_2}{(Py)_2}C{l_2}]^{2 + }}$
Answer
572.1k+ views
Hint: Isomers are compounds which have the same molecular formula but the arrangement of the atoms in the space is different. The physical and chemical properties of the isomers are not the same; they vary. The phenomenon of the existence of a compound in more than one form of isomers is known as isomerism.
The isomers are of different type’s functional, position, chain isomers. Chain isomers are the isomers which have the same molecular formula and are made up of carbon atoms but the atomic arrangement of these carbon atoms in space is different.
Complete step by step answer:
The isomerism which arises due to the restriction of rotation around a bond in the molecule is known as Geometrical isomerism. The E-Z or the Cis -Trans are the common form of the Geometrical isomerism. The restriction in the rotation is usually around the double bond i.e; carbon- carbon double bond (C=C). Geometrical isomers are the classic example of the compounds that include one or more metal atoms or ions and one or more ligands that formally donate electrons to the metal. Thus they are also regarded as the coordination compounds.The possible geometrical isomers of ${[Pt(IV){(N{H_3})_2}{(Py)_2}C{l_2}]^{2 + }}$are shown below:
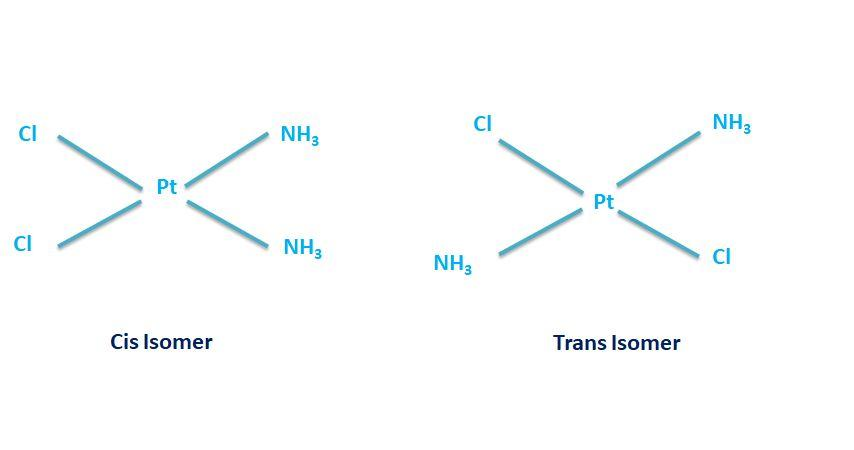
However no form among them is optically active due to the absence of the chirality in the molecule. The two forms are also not the mirror images of each other since new conformation will be there when they will be superimposed.
Note: Such compounds are frequently used in the treatment of the cancer and in the treatment of the tumours as well. Since they are usually inert in nature thus the complex is formed between the metal and the ligands and used in the treatment of the cancer.
The isomers are of different type’s functional, position, chain isomers. Chain isomers are the isomers which have the same molecular formula and are made up of carbon atoms but the atomic arrangement of these carbon atoms in space is different.
Complete step by step answer:
The isomerism which arises due to the restriction of rotation around a bond in the molecule is known as Geometrical isomerism. The E-Z or the Cis -Trans are the common form of the Geometrical isomerism. The restriction in the rotation is usually around the double bond i.e; carbon- carbon double bond (C=C). Geometrical isomers are the classic example of the compounds that include one or more metal atoms or ions and one or more ligands that formally donate electrons to the metal. Thus they are also regarded as the coordination compounds.The possible geometrical isomers of ${[Pt(IV){(N{H_3})_2}{(Py)_2}C{l_2}]^{2 + }}$are shown below:

However no form among them is optically active due to the absence of the chirality in the molecule. The two forms are also not the mirror images of each other since new conformation will be there when they will be superimposed.
Note: Such compounds are frequently used in the treatment of the cancer and in the treatment of the tumours as well. Since they are usually inert in nature thus the complex is formed between the metal and the ligands and used in the treatment of the cancer.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




