
How do you draw a chair conformation from a fischer projection?
Answer
560.7k+ views
Hint: Chair conformation is the most accurate form of representation for mainly rings. It tells appropriately about the angle between the carbon atoms in a ring and also tells about the position of the group on each carbon atom present in a ring.
Complete step by step answer:
- Fisher projections are very appropriate for drawing long chain molecules which have a huge number of chiral carbon, for example carbohydrates.
- The fischer projection restricts the molecule into a two-dimensional plan, such that the absolute configuration of the molecule remains unchanged.
- Also, depending on the number of stereo centres in the molecule, the total number of stereoisomers present can be determined.
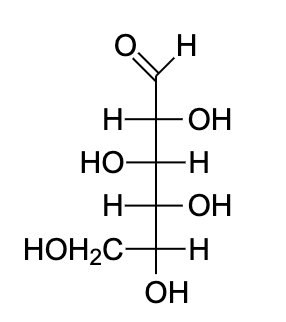
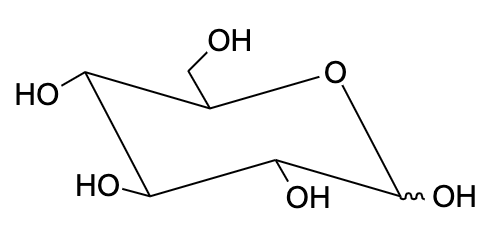
- Let us draw the Fischer projection of D-glucose. It is important to note that we need to change the position of the hydroxyl group on C-5 in the final orientation.
- This was important to understand the axial and equatorial bonds on carbon to determine which part goes up and which part goes down.
Note: The stability of chair conformation is found by adding up the A-value for each axial substituent. Lower the number, more stable the conformation is. The conformational isomers are the stereoisomers which differ in relative position of atoms within the molecule and which can be interconverted simply by rotation about the sigma bond. In this, the interconversion of the isomers does not require breaking and remaking of covalent bonds.
Complete step by step answer:
- Fisher projections are very appropriate for drawing long chain molecules which have a huge number of chiral carbon, for example carbohydrates.
- The fischer projection restricts the molecule into a two-dimensional plan, such that the absolute configuration of the molecule remains unchanged.
- Also, depending on the number of stereo centres in the molecule, the total number of stereoisomers present can be determined.
- Let us draw the Fischer projection of D-glucose. It is important to note that we need to change the position of the hydroxyl group on C-5 in the final orientation.
- This was important to understand the axial and equatorial bonds on carbon to determine which part goes up and which part goes down.


Note: The stability of chair conformation is found by adding up the A-value for each axial substituent. Lower the number, more stable the conformation is. The conformational isomers are the stereoisomers which differ in relative position of atoms within the molecule and which can be interconverted simply by rotation about the sigma bond. In this, the interconversion of the isomers does not require breaking and remaking of covalent bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




