
How can you distinguish the compound
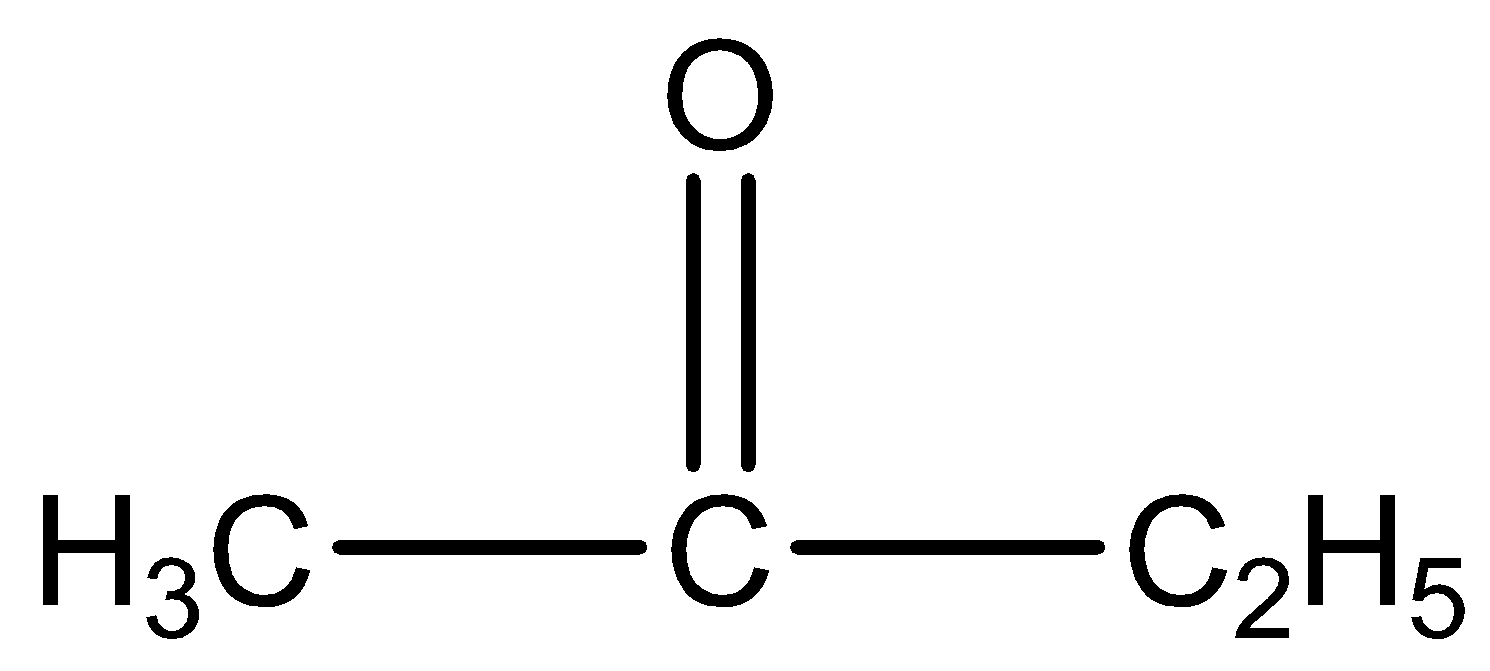 and
and
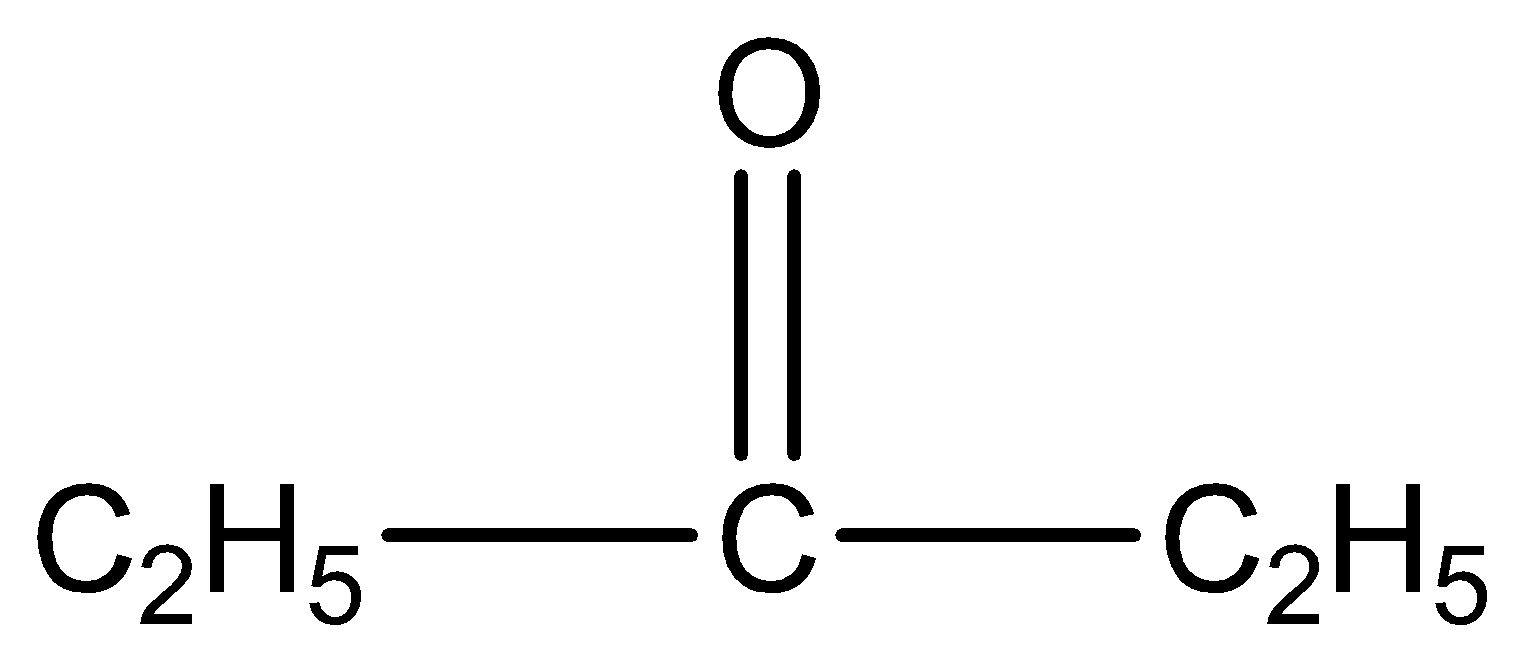 by a simple chemical test?
by a simple chemical test?


Answer
518.7k+ views
Hint: We must remember that organic analysis is important for qualification. In that organic analysis first we focused on the aromatic or non-aromatic compound. If it is aromatic, further study based on that weather benzenoid or non-benzenoid compound. If it is non-aromatic, further study goes to the acyclic or aliphatic compounds. Every compound functional analysis is very important.
Complete answer:
We also remember that the carbonyl is one of the classification of the organic analysis. In the carbonyl group further classified as aldehyde and ketone. The aldehyde and ketone group are separated by Tollens reagent or mirror test. If aldehyde present in the given compound means carbonyl test and Tollens reagent test both are positive results. If it is ketone means the carbonyl test will have a positive result but the mirror test will be negative.
In ketone further classified as methyl and non-methyl ketone. This methyl ketone is identified by Iodoform test.
The compound is treated with sodium hydroxide and in presence of iodine molecules. It reacts with compounds and forms Iodoform as one of the products. This iodoform is detected by the yellow precipitate that will form in the product.
If it is non-methyl ketone means Iodoform will not form and no yellow precipitate is formed.
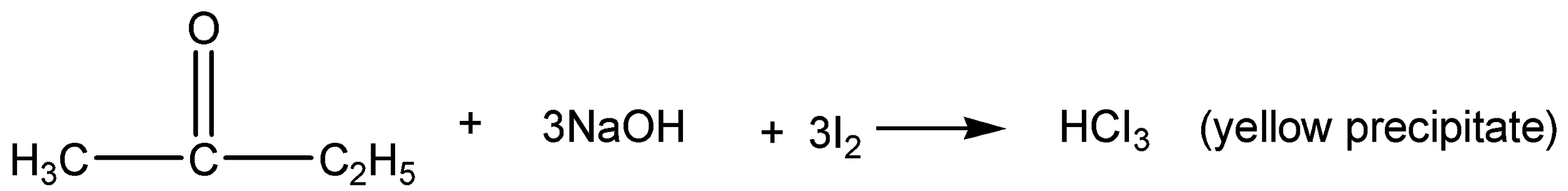
This give positive result because it has a methyl group ketone.
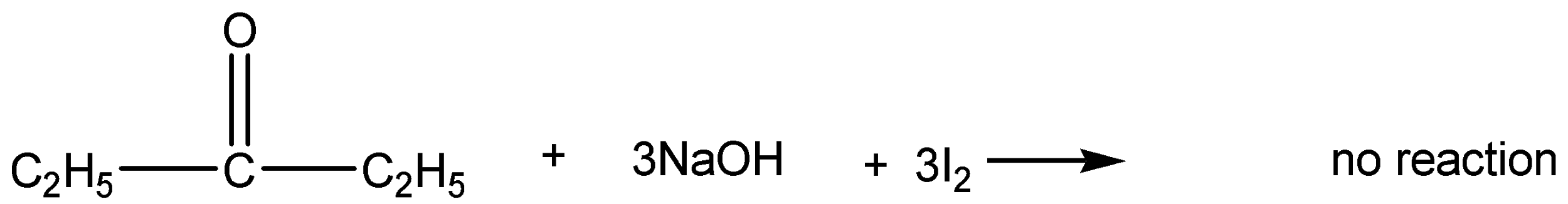
This gives a negative result because it doesn’t have methyl group ketone.
Iodoform is best test for distinguish
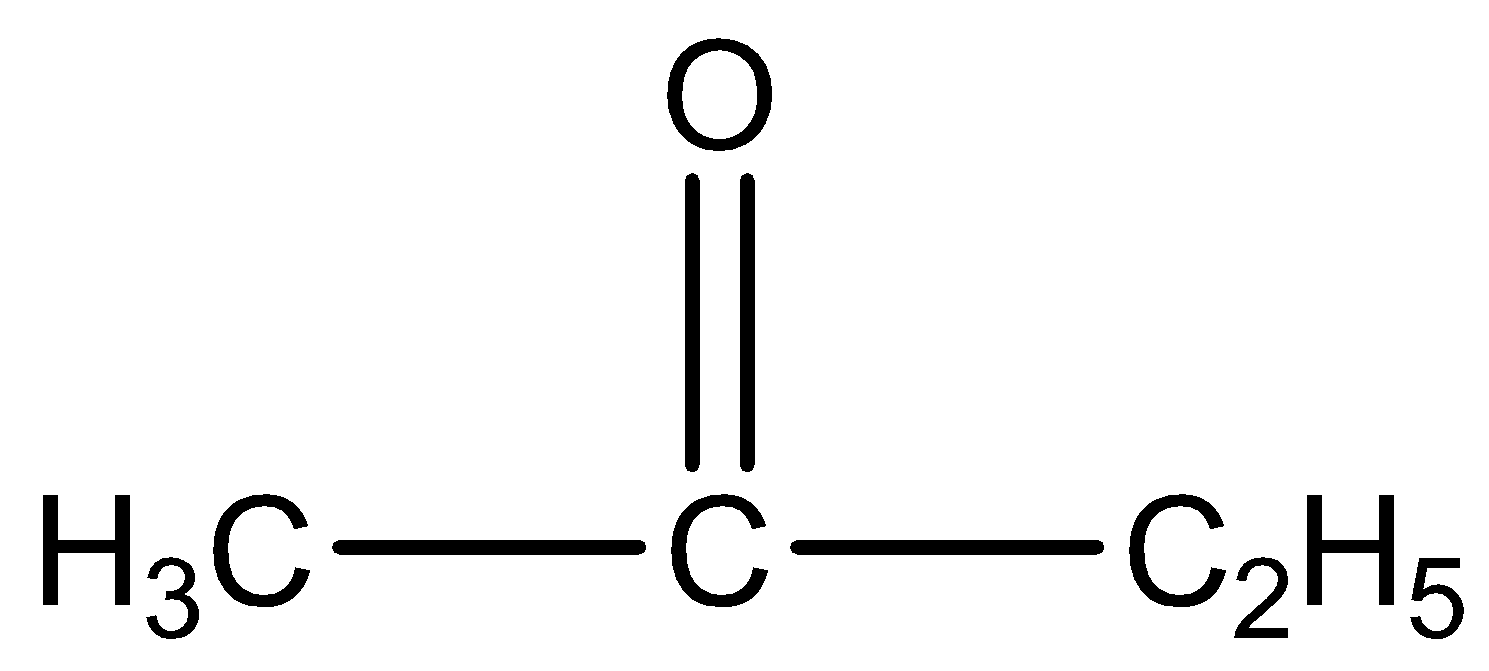 and
and
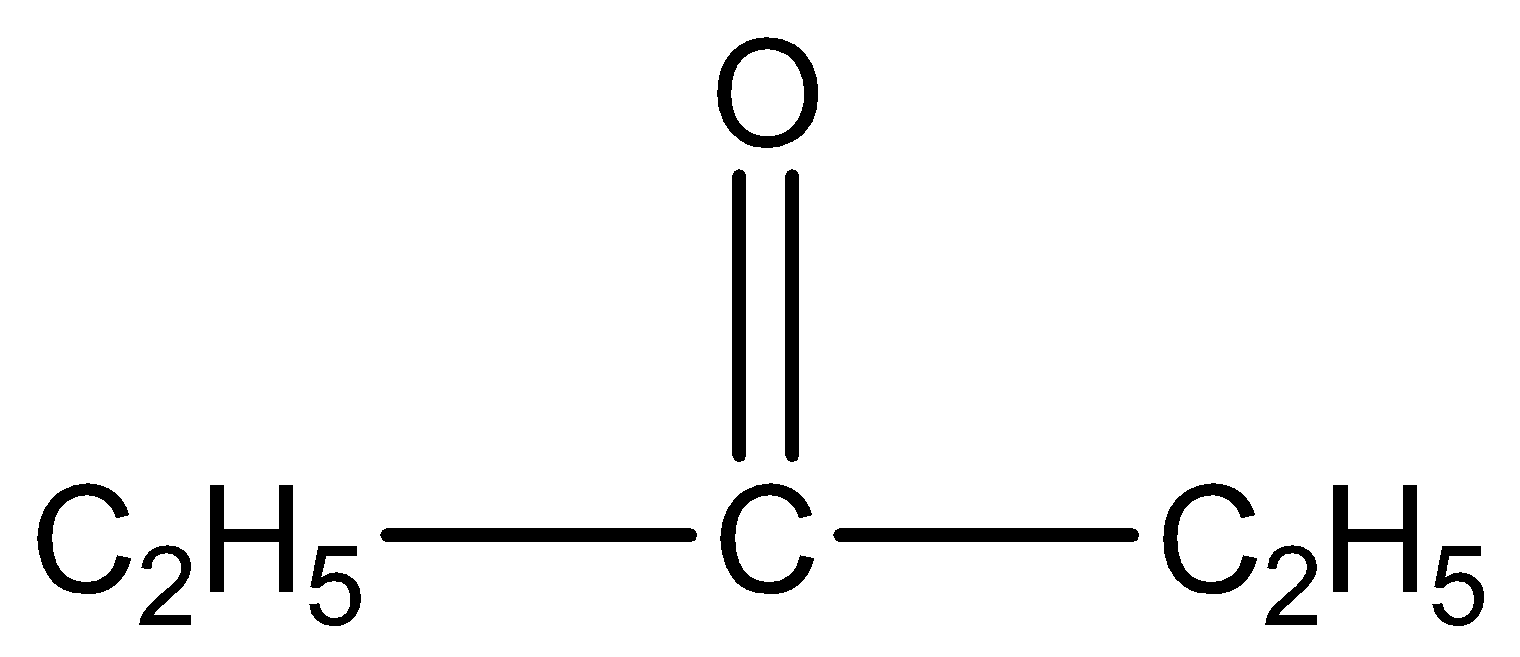 .
.
Note:
The IUPAC name of the given compounds are given below. The IUPAC name of
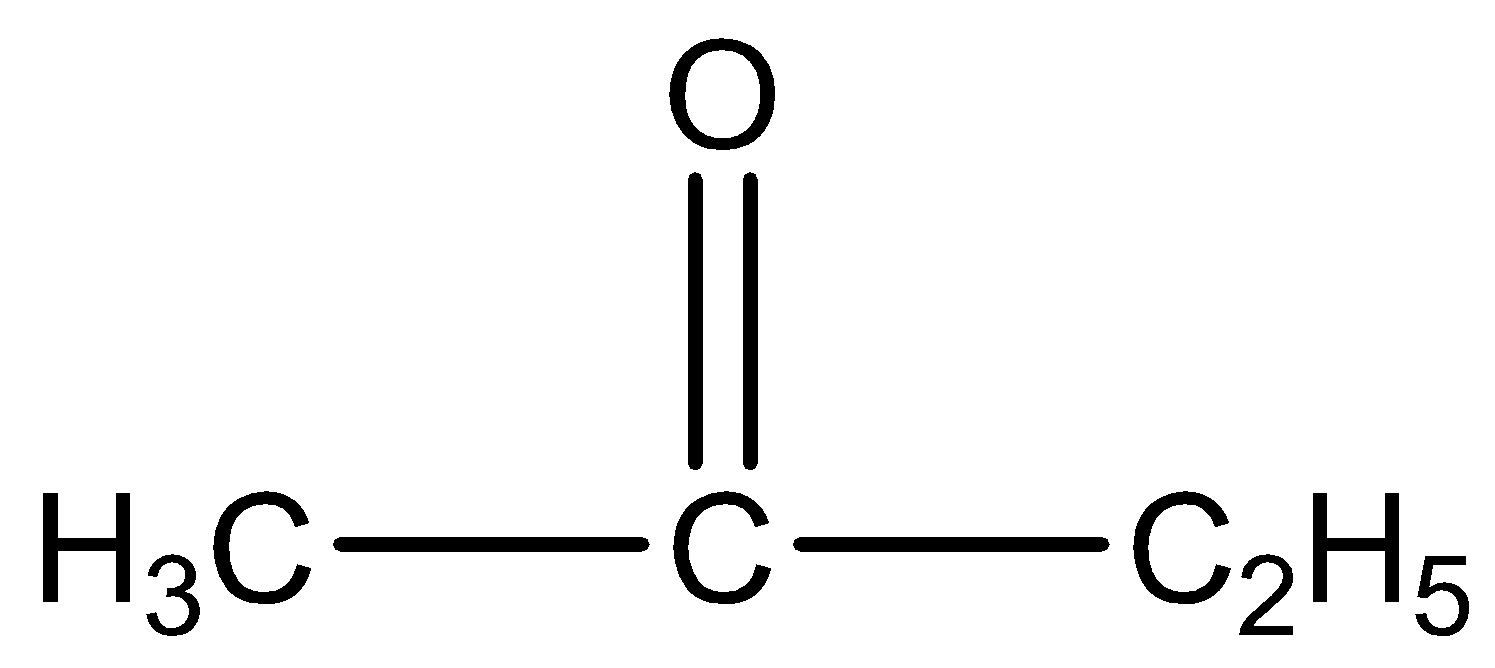 is butanone. The IUPAC name of
is butanone. The IUPAC name of
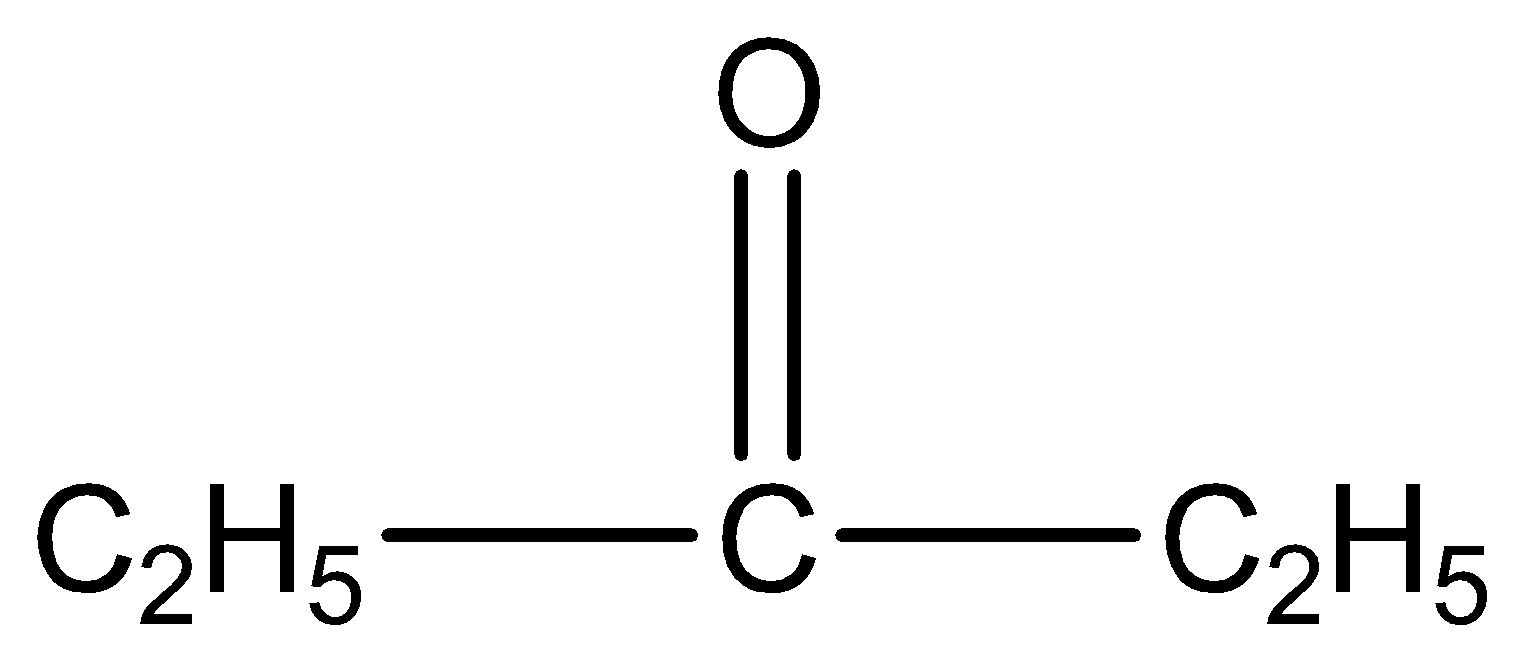 is pentan-3-one. The number three represents the position of the functional group in the molecule.
is pentan-3-one. The number three represents the position of the functional group in the molecule.
The functional group present in the molecule means it is ended with that means the suffix of the name will be one. Depending on the functional group present in the molecule the suffix of the name will be changed. If more than one functional group present in the molecule depends on the priority of the functional group in the molecule.
Complete answer:
We also remember that the carbonyl is one of the classification of the organic analysis. In the carbonyl group further classified as aldehyde and ketone. The aldehyde and ketone group are separated by Tollens reagent or mirror test. If aldehyde present in the given compound means carbonyl test and Tollens reagent test both are positive results. If it is ketone means the carbonyl test will have a positive result but the mirror test will be negative.
In ketone further classified as methyl and non-methyl ketone. This methyl ketone is identified by Iodoform test.
The compound is treated with sodium hydroxide and in presence of iodine molecules. It reacts with compounds and forms Iodoform as one of the products. This iodoform is detected by the yellow precipitate that will form in the product.
If it is non-methyl ketone means Iodoform will not form and no yellow precipitate is formed.
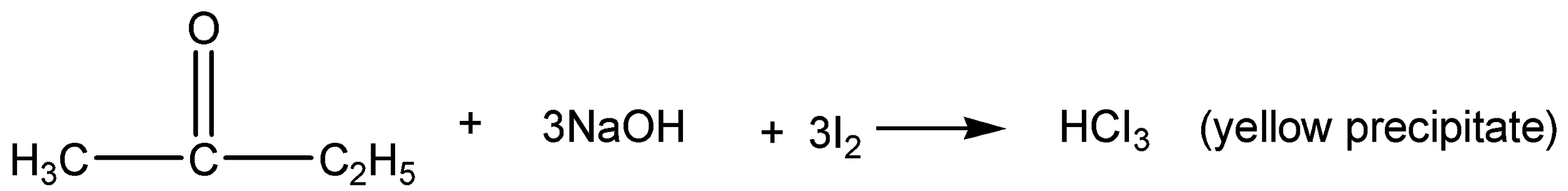
This give positive result because it has a methyl group ketone.
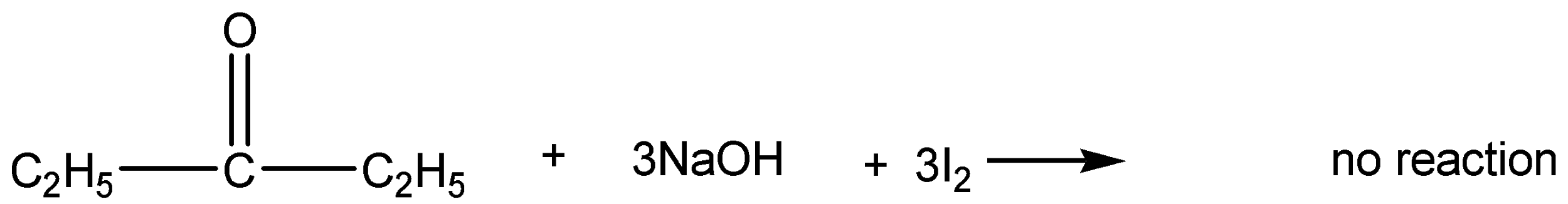
This gives a negative result because it doesn’t have methyl group ketone.
Iodoform is best test for distinguish


Note:
The IUPAC name of the given compounds are given below. The IUPAC name of


The functional group present in the molecule means it is ended with that means the suffix of the name will be one. Depending on the functional group present in the molecule the suffix of the name will be changed. If more than one functional group present in the molecule depends on the priority of the functional group in the molecule.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

What is the difference between biodegradable and nonbiodegradable class 11 biology CBSE

Proton was discovered by A Thomson B Rutherford C Chadwick class 11 chemistry CBSE




