
Discuss the hybridization in case of \[PC{l_5}\]. Why are the axial bonds longer as compared to equatorial bonds?
Answer
594.6k+ views
Hint: Phosphorus is an element of nitrogen group and ground state electronic configuration of Phosphorus atom is \[[Ne]3{s^2}3{p^3}\]. Repulsive forces may make some bonds longer due to bond pair-bond pair repulsion.
Complete answer:
We know that ground state electronic configuration of Phosphorus atom is \[[Ne]3{s^2}3{p^3}\] and excited state electronic configuration is \[[Ne]3{s^1}3{p^3}3{d^1}\]. It is shown below.
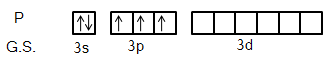
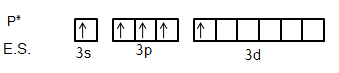
So, each chlorine atom will donate one electron each to each hybrid orbitals and for new five sigma bonds. So, we can see that there are five orbitals involved in the hybridization and the orbitals are one s, three p and one d-orbital. So, we can say that the molecule has \[s{p^3}d\] hybridization. This molecule favours the Pentagonal bipyramidal shape. Let’s see the shape of the molecule first.
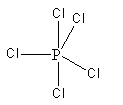
As you can see that three chlorine atoms are in one plane and the other two are forming a perpendicular line to the plane.
Here, the chlorine atoms that are present in the plane are called equatorial atoms and the atoms that are in perpendicular line, are called axial atoms.
Note, these axial atoms feel more repulsive forces with three adjacent chlorine atoms, hence they try to be as far as possible from the equatorial chlorine atoms.
That is the reason why the axial bonds are longer compared to equatorial bonds.
Additional Information:
- In \[s{p^3}d\] hybridization, compound can have either trigonal bipyramidal or square pyramidal geometry.
- If a compound is having trigonal bipyramidal geometry, then it uses \[{d_{{z^2}}}\] orbital as one of the d-orbitals to form bonds. If a compound has square pyramidal geometry, then \[{d_{{x^2} - {y^2}}}\] is used as one of the d-orbitals to make bonds.
Note: Note that \[ds{p^3}\] is different from \[s{p^3}d\] hybridization. So, do not get confused between them. One needs to remember that \[PC{l_5}\] does not have square bipyramidal geometry as it is also a possible geometry for compounds that have \[s{p^3}d\] hybridization.
Complete answer:
We know that ground state electronic configuration of Phosphorus atom is \[[Ne]3{s^2}3{p^3}\] and excited state electronic configuration is \[[Ne]3{s^1}3{p^3}3{d^1}\]. It is shown below.


So, each chlorine atom will donate one electron each to each hybrid orbitals and for new five sigma bonds. So, we can see that there are five orbitals involved in the hybridization and the orbitals are one s, three p and one d-orbital. So, we can say that the molecule has \[s{p^3}d\] hybridization. This molecule favours the Pentagonal bipyramidal shape. Let’s see the shape of the molecule first.

As you can see that three chlorine atoms are in one plane and the other two are forming a perpendicular line to the plane.
Here, the chlorine atoms that are present in the plane are called equatorial atoms and the atoms that are in perpendicular line, are called axial atoms.
Note, these axial atoms feel more repulsive forces with three adjacent chlorine atoms, hence they try to be as far as possible from the equatorial chlorine atoms.
That is the reason why the axial bonds are longer compared to equatorial bonds.
Additional Information:
- In \[s{p^3}d\] hybridization, compound can have either trigonal bipyramidal or square pyramidal geometry.
- If a compound is having trigonal bipyramidal geometry, then it uses \[{d_{{z^2}}}\] orbital as one of the d-orbitals to form bonds. If a compound has square pyramidal geometry, then \[{d_{{x^2} - {y^2}}}\] is used as one of the d-orbitals to make bonds.
Note: Note that \[ds{p^3}\] is different from \[s{p^3}d\] hybridization. So, do not get confused between them. One needs to remember that \[PC{l_5}\] does not have square bipyramidal geometry as it is also a possible geometry for compounds that have \[s{p^3}d\] hybridization.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




