
Dipole moment is shown by: (This question has multiple correct options)
A.1, 4-dichlorobenzene
B.cis-1, 2-dichloro ethene
C.trans-1, 2-dichloroethene
D.trans-2, 3-dichloro-2-butene
Answer
609k+ views
Hint: To answer this question, we should first draw the structures of these structures and then we will decide which structure shows dipole moment. We should use the concept that the larger the difference in electronegativities of bonded atoms, the larger the dipole moment.
Step by step solution:
First we should understand that whenever there is separation of charge, there will be a dipole moment. Dipole moment arises in a compound whenever there is difference in electronegativity. We should know that the larger the difference in electronegativity, the larger the dipole moment. The distance between the charge separations is also a deciding factor into the size of the dipole moment. The dipole moment is a measure of the polarity of the molecule.
So, we should first define the dipole moment properly. We should know that when two electrical charges, of opposite sign and equal magnitude, are separated by a distance, an electric dipole is established. We measure the size of dipole moment by ( μ ).
So, now one by one we will draw the structures of compounds that are present in the option to check whether the options will show dipole moment or not.
Let’s take a look at the first option of 1, 4-dichlorobenzene.
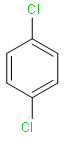
1, 4-dichlorobenzene will have no dipole moment, because it has symmetry. Chlorine groups are attached opposite sides that are why they will cancel each other dipole moments and hence they have zero dipole moment.
Our second option is Cis-1, 2-dichloroethene. Its structure is as follows:
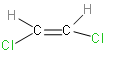
Cis isomer has more dipole moment than trans isomer, because it has two similar groups on same side of double bond. So the dipole gets added, and hence cis isomer is more polar than trans. Hence, it has a dipole moment.
Our third option is trans-1, 2-dichloroethene.

We know that the above structure is a trans isomer. We should know that in Trans isomer, there is a Centre of Symmetry, located in the middle of the C = C bond. When we consider, for example, the contribution of the vector corresponding to the dipole moment of one of the C - Cl bonds, there will be an identical vectorial contribution from the other C - Cl polar bond, but now pointed in the opposite direction: the net contribution to the Dipole Moment of the molecule will be zero.
Now, we will see the fourth option that is of trans-2, 3-dichloro-2-butene. Let us look at the structure of this:

As we know the above structure is also of trans isomer, so it also has a centre of symmetry and that’s why it also doesn’t have any dipole moment.
So, from the above discussion we came to know that option B, which is cis-1, 2-dichloro ethene will show dipole moment.
Note: We should note that other factors like shape of molecule and the polarity of its bonds determine the polarity of compounds. We should know that if the centers of molecules lie at the same point in space, then the molecule has no overall polarity and it will be called non-polar. If a molecule is completely symmetric, then the dipole moment vectors on each molecule will cancel each other out, making the molecule nonpolar. We should call a molecule polar only if the structure of that molecule is not symmetric.
Step by step solution:
First we should understand that whenever there is separation of charge, there will be a dipole moment. Dipole moment arises in a compound whenever there is difference in electronegativity. We should know that the larger the difference in electronegativity, the larger the dipole moment. The distance between the charge separations is also a deciding factor into the size of the dipole moment. The dipole moment is a measure of the polarity of the molecule.
So, we should first define the dipole moment properly. We should know that when two electrical charges, of opposite sign and equal magnitude, are separated by a distance, an electric dipole is established. We measure the size of dipole moment by ( μ ).
So, now one by one we will draw the structures of compounds that are present in the option to check whether the options will show dipole moment or not.
Let’s take a look at the first option of 1, 4-dichlorobenzene.

1, 4-dichlorobenzene will have no dipole moment, because it has symmetry. Chlorine groups are attached opposite sides that are why they will cancel each other dipole moments and hence they have zero dipole moment.
Our second option is Cis-1, 2-dichloroethene. Its structure is as follows:
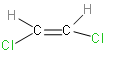
Cis isomer has more dipole moment than trans isomer, because it has two similar groups on same side of double bond. So the dipole gets added, and hence cis isomer is more polar than trans. Hence, it has a dipole moment.
Our third option is trans-1, 2-dichloroethene.

We know that the above structure is a trans isomer. We should know that in Trans isomer, there is a Centre of Symmetry, located in the middle of the C = C bond. When we consider, for example, the contribution of the vector corresponding to the dipole moment of one of the C - Cl bonds, there will be an identical vectorial contribution from the other C - Cl polar bond, but now pointed in the opposite direction: the net contribution to the Dipole Moment of the molecule will be zero.
Now, we will see the fourth option that is of trans-2, 3-dichloro-2-butene. Let us look at the structure of this:

As we know the above structure is also of trans isomer, so it also has a centre of symmetry and that’s why it also doesn’t have any dipole moment.
So, from the above discussion we came to know that option B, which is cis-1, 2-dichloro ethene will show dipole moment.
Note: We should note that other factors like shape of molecule and the polarity of its bonds determine the polarity of compounds. We should know that if the centers of molecules lie at the same point in space, then the molecule has no overall polarity and it will be called non-polar. If a molecule is completely symmetric, then the dipole moment vectors on each molecule will cancel each other out, making the molecule nonpolar. We should call a molecule polar only if the structure of that molecule is not symmetric.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

Which out of the following hydrocarbons undergo addition class 11 chemistry CBSE




