
Dihalogen derivative (A) of a hydrocarbon having two carbon atoms reacts with alcoholic potash and forms another hydrocarbon which gives a red precipitate with ammoniacal cuprous chloride. Compound A gives an aldehyde when treated with aqueous KOH. Write down the name and formula for the organic compound.
Answer
590.7k+ views
Hint: When we boil the geminal dihalides with \[aq.{\text{ }}KOH\] or \[aq.NaOH\]. It gives aldehydes and ketones as a product. So we can use the above information to solve this question.
Complete step by step answer:
Following is the summary of question,
\[Dihalogen{\text{ }}derivative\left( {2C} \right) + alcoholic\;potash \to hydrocarbon{\text{ }}product\] ………………………………. (1)
From equation 1,
Hydrocarbon product + Ammoniacal cuprous chloride → substance that forms Red precipitate
Again from equation 1,
\[Dihalogen{\text{ }}derivative\left( {2C} \right) + aq.KOH \to Aldehyde\]
And it has been asked to find the name and formula of dihalogen derivative.
So, the compound A is the dihalogen derivative of hydrocarbon. Again it has mentioned that the compound is a 2-carbon compound. So, let’s assume that the two carbon containing hydrocarbons with two halogen group are geminal dihalides \[\left( {C{H_3} - CH - {X_2}} \right)\] having two halogen atoms situated on a terminal carbon atom.
So, considering halogen X as chlorine, the \[C{H_3} - CH - {X_2}\] is 1-1- dichloroethane, which is geminal dihalides that react with KOH and produce an unstable hydroxyl compound. This hydroxyl compound on water molecule loss yields acetaldehyde \[\left( {C{H_3} - CHO} \right)\]
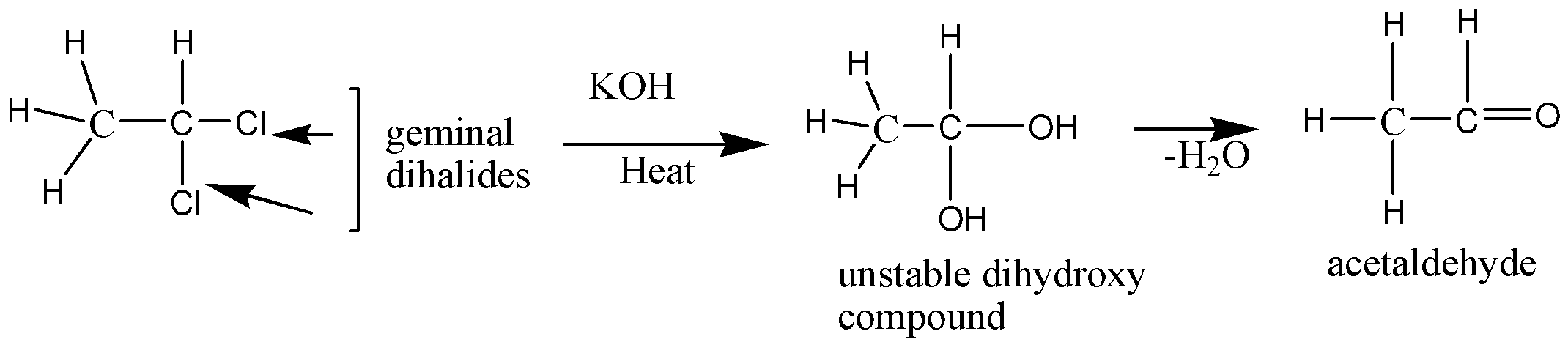
This geminal dihalide of 1-1- dichloroethane when reacts with alcoholic \[KOH\] or potash undergoes the dehydrohalogenation process and yields \[CH \equiv CH\], acetylene or ethyne
Acetylene or Ethyne in presence of ammoniacal cuprous chloride forms a red-brown colored precipitate of 1,3-Butyne.
So, Compound A is 1,1 dichloroethane whose chemical formula is \[C{H_3} - CH - C{l_2}\]
Note:
We must know that the Glaser coupling test is the identification test for terminal alkynes, where terminal alkyne reacts with ammoniacal cupric chloride which on subsequent oxidation in air gives di-yne.
Complete step by step answer:
Following is the summary of question,
\[Dihalogen{\text{ }}derivative\left( {2C} \right) + alcoholic\;potash \to hydrocarbon{\text{ }}product\] ………………………………. (1)
From equation 1,
Hydrocarbon product + Ammoniacal cuprous chloride → substance that forms Red precipitate
Again from equation 1,
\[Dihalogen{\text{ }}derivative\left( {2C} \right) + aq.KOH \to Aldehyde\]
And it has been asked to find the name and formula of dihalogen derivative.
So, the compound A is the dihalogen derivative of hydrocarbon. Again it has mentioned that the compound is a 2-carbon compound. So, let’s assume that the two carbon containing hydrocarbons with two halogen group are geminal dihalides \[\left( {C{H_3} - CH - {X_2}} \right)\] having two halogen atoms situated on a terminal carbon atom.
So, considering halogen X as chlorine, the \[C{H_3} - CH - {X_2}\] is 1-1- dichloroethane, which is geminal dihalides that react with KOH and produce an unstable hydroxyl compound. This hydroxyl compound on water molecule loss yields acetaldehyde \[\left( {C{H_3} - CHO} \right)\]

This geminal dihalide of 1-1- dichloroethane when reacts with alcoholic \[KOH\] or potash undergoes the dehydrohalogenation process and yields \[CH \equiv CH\], acetylene or ethyne
Acetylene or Ethyne in presence of ammoniacal cuprous chloride forms a red-brown colored precipitate of 1,3-Butyne.
So, Compound A is 1,1 dichloroethane whose chemical formula is \[C{H_3} - CH - C{l_2}\]
Note:
We must know that the Glaser coupling test is the identification test for terminal alkynes, where terminal alkyne reacts with ammoniacal cupric chloride which on subsequent oxidation in air gives di-yne.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




