
How many dichloro products are formed in the above reaction (including stereoisomers)?
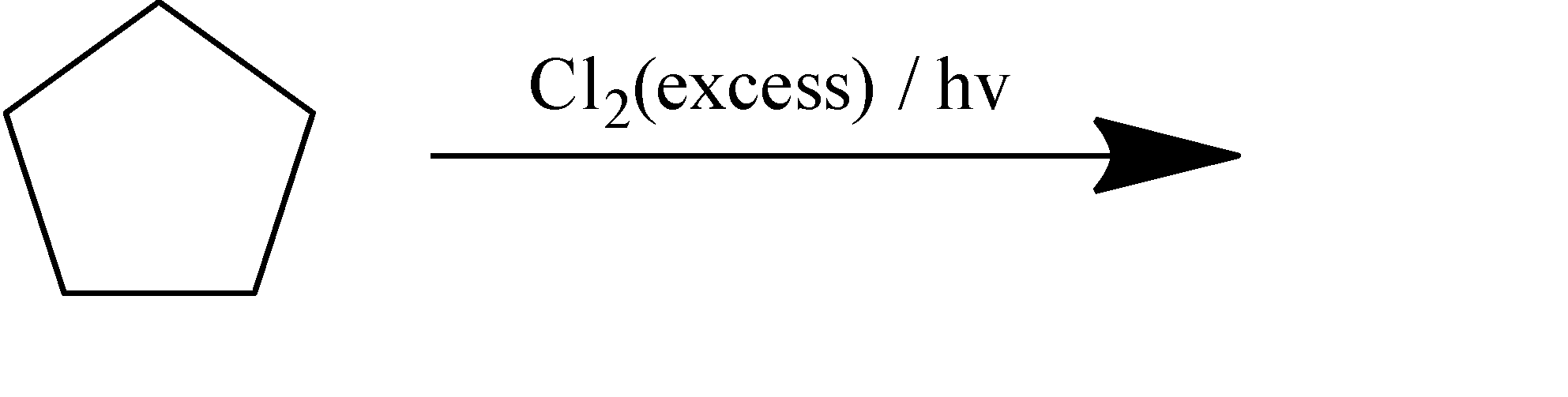
A.5
B.6
C.7
D.9
Answer
558.9k+ views
Hint: The chemical compounds are formed by the difference in the arrangement of the constituent atoms in the compound. Some of the compounds have the same chemical formula but the arrangement of the atoms in the compound is different. These types of compounds are called isomers.
Complete step by step answer:
The compounds which are different in just the structure but have the same formula are called isomers, some of the isomers can show optical activity, these are called optical isomerism.
When the same compound has a different spatial arrangement in the space, then that is called stereoisomers.
One type of isomerism is cis-trans isomerism. In this type of isomerism, the group is attached either to the same side of the double bond or the different sides to the double bond. If the groups are on the same side of the double bond then these are called the cis type isomer and if the groups are on the opposite side of the double bond then they are called a trans isomer.
Although this type of isomerism is mostly found in the double bond it may not be confused to be only limited to that. the actual meaning of cis-trans rises to the orientation of the bonds in space. One bond faces forward towards us while the other faces away or backward.
When cyclopentane goes chlorination, the chlorine atom gets attached to all the possible positions of the cyclic compound. If we were to name according to the IUPAC conventions, the following three structures come out as distinct dichlorinated products. After the addition of the first chlorine, there are only three places for the other chlorine atom to be attached, which are the same atom, adjacent carbon, and the next to the adjacent atom.
The reaction results in the cis, trans variations in the first two structures but the third structure doesn't allow two isomers due to the bond repulsion and steric hindrance.
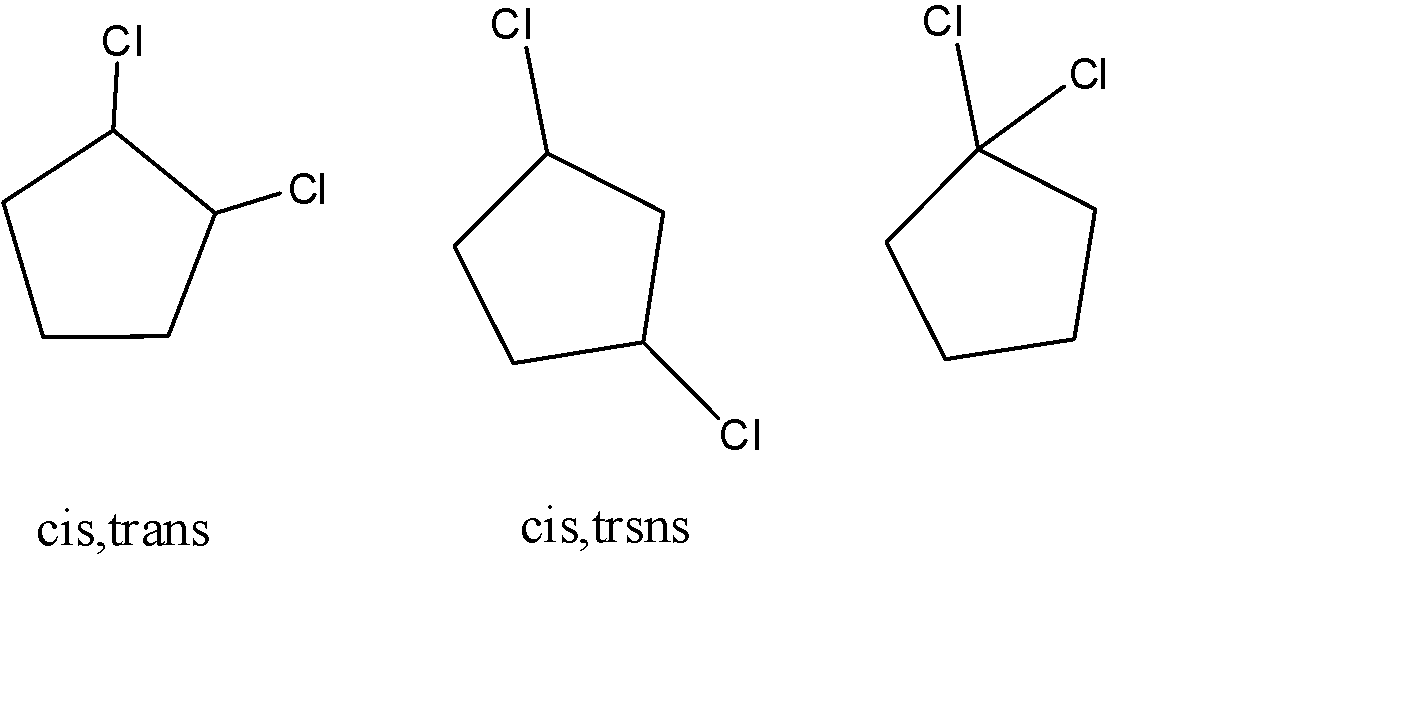
The total number of dichlorinated products will be 5. The first and second structures will produce cis and trans isomers and thus the count of the compounds formed will be 5.
So the correct answer is A.
Note: The naming convention of Z and E can also be used in the place of the cis-trans naming. Here Z is used in place of cis type isomer and E is used in place of trans type isomer.
E stands for Entagagen which means opposite and Z stands for Zussamen which means together.
Complete step by step answer:
The compounds which are different in just the structure but have the same formula are called isomers, some of the isomers can show optical activity, these are called optical isomerism.
When the same compound has a different spatial arrangement in the space, then that is called stereoisomers.
One type of isomerism is cis-trans isomerism. In this type of isomerism, the group is attached either to the same side of the double bond or the different sides to the double bond. If the groups are on the same side of the double bond then these are called the cis type isomer and if the groups are on the opposite side of the double bond then they are called a trans isomer.
Although this type of isomerism is mostly found in the double bond it may not be confused to be only limited to that. the actual meaning of cis-trans rises to the orientation of the bonds in space. One bond faces forward towards us while the other faces away or backward.
When cyclopentane goes chlorination, the chlorine atom gets attached to all the possible positions of the cyclic compound. If we were to name according to the IUPAC conventions, the following three structures come out as distinct dichlorinated products. After the addition of the first chlorine, there are only three places for the other chlorine atom to be attached, which are the same atom, adjacent carbon, and the next to the adjacent atom.
The reaction results in the cis, trans variations in the first two structures but the third structure doesn't allow two isomers due to the bond repulsion and steric hindrance.

The total number of dichlorinated products will be 5. The first and second structures will produce cis and trans isomers and thus the count of the compounds formed will be 5.
So the correct answer is A.
Note: The naming convention of Z and E can also be used in the place of the cis-trans naming. Here Z is used in place of cis type isomer and E is used in place of trans type isomer.
E stands for Entagagen which means opposite and Z stands for Zussamen which means together.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




