
How can you determine whether a molecule is achiral or chiral?
Answer
552k+ views
Hint:We know that the chiral carbon is carbon in which all four valence of carbon is satisfied by different elements. If even one of valence is satisfied by different elements then carbon is said to be achiral. So first we need to check if there is chiral carbon present in the given compound.
Complete step-by-step answer:Firstly we need to find in which compound is achiral.
1.Chirality is more commonly observed in complex molecules than achirality.
2.Achiral objects show identical mirror images to actual objects.
3.Chiral objects don’t show an identical mirror image to the actual object because of asymmetrical configuration of object/item.
4.Both achiral as well as chiral objects can show rotational symmetry around $360$ degrees but chiral objects do not have reflective symmetry.
5.Chirality is an important aspect of chemistry since different enantiomers may have different reactivities.
6.Knowledge of chirality of molecular structure can help in treatment of diseases along with design of medication.
The compound they don’t have chiral carbon will be achiral molecule/we can say compound. So first we will check if there is chiral carbon present with the given option one another by one.
1.First compound we take for example is \[butan-2-ol\]
\[C{{H}_{3}}-CH\left( OH \right)-C{{H}_{2}}-C{{H}_{3}}\]whose structure is given as:
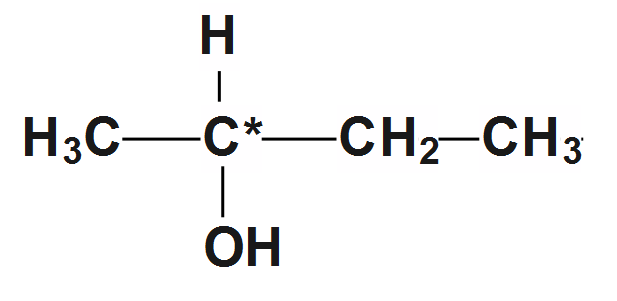
Now here we can see in structure that the second carbon is a chiral compound as all four valences are satisfied by the different elements. It is a chiral center as well as it is indicated by an asterisk sign. So it's not achiral.
2.Here the second compound is a \[Pentane-1,3-diol\] Therefore the structure is given as:
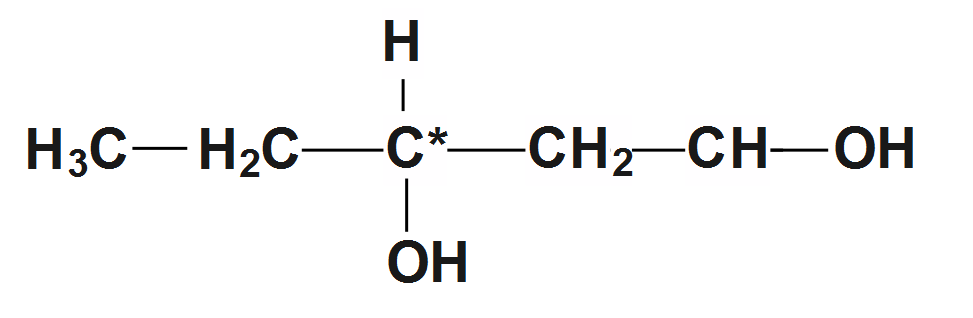
So we can see in the structure that carbon in the center has different elements attached to it. So it is a chiral compound.
Note:We can also find achiral compound by these methods:
1.First we have to draw a mirror image of the compound, then we see if two molecules are the same or different.
2.If the mirror image of a compound is the same as the image of a compound then it's chiral.
3.If the mirror image of compounds is different then it's achiral.
Complete step-by-step answer:Firstly we need to find in which compound is achiral.
1.Chirality is more commonly observed in complex molecules than achirality.
2.Achiral objects show identical mirror images to actual objects.
3.Chiral objects don’t show an identical mirror image to the actual object because of asymmetrical configuration of object/item.
4.Both achiral as well as chiral objects can show rotational symmetry around $360$ degrees but chiral objects do not have reflective symmetry.
5.Chirality is an important aspect of chemistry since different enantiomers may have different reactivities.
6.Knowledge of chirality of molecular structure can help in treatment of diseases along with design of medication.
The compound they don’t have chiral carbon will be achiral molecule/we can say compound. So first we will check if there is chiral carbon present with the given option one another by one.
1.First compound we take for example is \[butan-2-ol\]
\[C{{H}_{3}}-CH\left( OH \right)-C{{H}_{2}}-C{{H}_{3}}\]whose structure is given as:

Now here we can see in structure that the second carbon is a chiral compound as all four valences are satisfied by the different elements. It is a chiral center as well as it is indicated by an asterisk sign. So it's not achiral.
2.Here the second compound is a \[Pentane-1,3-diol\] Therefore the structure is given as:

So we can see in the structure that carbon in the center has different elements attached to it. So it is a chiral compound.
Note:We can also find achiral compound by these methods:
1.First we have to draw a mirror image of the compound, then we see if two molecules are the same or different.
2.If the mirror image of a compound is the same as the image of a compound then it's chiral.
3.If the mirror image of compounds is different then it's achiral.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




