
Describe the purification of a colloidal solution by dialysis method with a neat diagram
Answer
583.5k+ views
Hint: The process of separating the particles of colloid from those of crystalloid, using diffusion through a suitable membrane is called dialysis. The process in which the ions are removed from the solution by the phenomenon of diffusion through a permeable membrane is also known as dialysis.
Complete step by step answer:
The diagrammatic representation of the dialysis process is as follows:
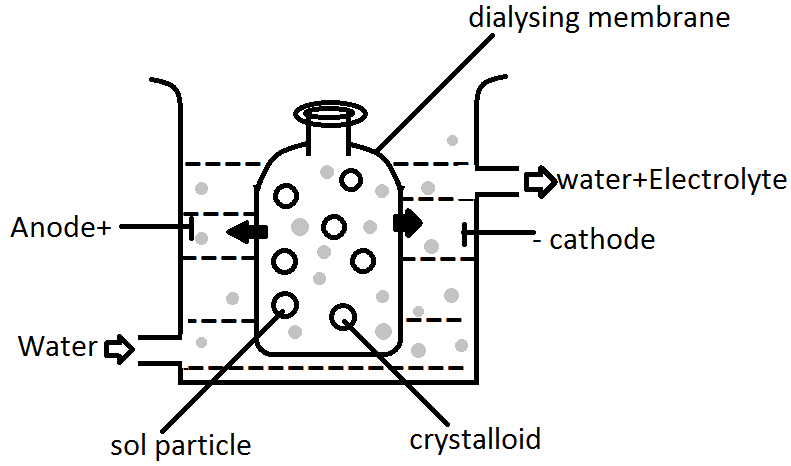
In the above diagram we can see that pure water is inserted in the container and the mixture of water and electrolyte is allowed to move out in the presence of electrical current or we can say voltage supplied.
Purification of a colloidal solution by Dialysis method →
It is the process of separating colloidal particles from those of crystalloids by diffusing the mixture through a parchment of or animal membrane.
Colloidal particles do not pass through the membrane while those of crystalloids do.
The impure sol is filled into a cellophane bag, which is suspended in a vessel containing distilled water. The crystalloid particle passes through the membrane while colloidal particles do not.
In this process, a sol consisting of ions or molecules is filled in a permeable membrane bag, dipped in the water. The ion from the solution diffuses through the permeable membrane.
Because of the continuous flow of water, the concentration of electrolyte outside the membrane is neutralized. Hence this is the whole process of purification of a colloidal solution.
Note:
This principle is based upon the fact that colloidal particles can not pass through a parchment or cellophane membrane while the ions of the electrolyte can pass through it.The impurities slowly diffused out of the bag leaving behind a pure colloidal solution.
Complete step by step answer:
The diagrammatic representation of the dialysis process is as follows:

In the above diagram we can see that pure water is inserted in the container and the mixture of water and electrolyte is allowed to move out in the presence of electrical current or we can say voltage supplied.
Purification of a colloidal solution by Dialysis method →
It is the process of separating colloidal particles from those of crystalloids by diffusing the mixture through a parchment of or animal membrane.
Colloidal particles do not pass through the membrane while those of crystalloids do.
The impure sol is filled into a cellophane bag, which is suspended in a vessel containing distilled water. The crystalloid particle passes through the membrane while colloidal particles do not.
In this process, a sol consisting of ions or molecules is filled in a permeable membrane bag, dipped in the water. The ion from the solution diffuses through the permeable membrane.
Because of the continuous flow of water, the concentration of electrolyte outside the membrane is neutralized. Hence this is the whole process of purification of a colloidal solution.
Note:
This principle is based upon the fact that colloidal particles can not pass through a parchment or cellophane membrane while the ions of the electrolyte can pass through it.The impurities slowly diffused out of the bag leaving behind a pure colloidal solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




