
Describe the construction, working and usefulness of a dry cell, with the help of a diagram.
Answer
564.9k+ views
Hint: You could recall what exactly a dry cell is. Then you could explain the basic structure of this dry cell and you could make a neat diagram of this cell. You could then describe its working without going deeply into the chemical reaction part. Also, mention the use of a dry cell.
Complete solution:
In the question, we asked to describe the construction, working and also the usefulness of a dry cell with the help of a diagram.
Basically, a dry cell could be categorized as a type of electric battery that can be commonly used for portable electrical devices. A commonly used dry cell is the zinc carbon cell or the dry Leclanche cell.
The basic structure of a Leclanche cell consists of a zinc anode and a carbon cathode. It uses ammonium chloride as electrolyte in the form of paste. There is also a second paste present in the cell which is a mixture of ammonium chloride and manganese dioxide where manganese dioxide acts as a depolarizer.
The basic structure of a dry cell is given by,
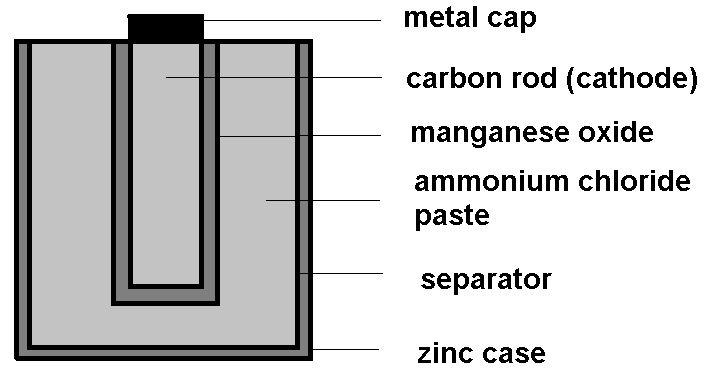
The working of this cell is based on the chemical reactions that take place in it. As the result of these reaction electrons are being built up at the anode which further results in potential difference between the cathode and the anode. As a result of this potential difference the electrons are able to flow through the circuit.
These are the batteries used in portable electrical devices.
Note:
The dry cells are further categorized into primary cells and secondary cells. The primary cells are not rechargeable, that is, the chemicals present in them get used up and cannot be used after that. So they are disposed of in such a condition. Secondary cells are known to be rechargeable and thus can be used many times.
Complete solution:
In the question, we asked to describe the construction, working and also the usefulness of a dry cell with the help of a diagram.
Basically, a dry cell could be categorized as a type of electric battery that can be commonly used for portable electrical devices. A commonly used dry cell is the zinc carbon cell or the dry Leclanche cell.
The basic structure of a Leclanche cell consists of a zinc anode and a carbon cathode. It uses ammonium chloride as electrolyte in the form of paste. There is also a second paste present in the cell which is a mixture of ammonium chloride and manganese dioxide where manganese dioxide acts as a depolarizer.
The basic structure of a dry cell is given by,

The working of this cell is based on the chemical reactions that take place in it. As the result of these reaction electrons are being built up at the anode which further results in potential difference between the cathode and the anode. As a result of this potential difference the electrons are able to flow through the circuit.
These are the batteries used in portable electrical devices.
Note:
The dry cells are further categorized into primary cells and secondary cells. The primary cells are not rechargeable, that is, the chemicals present in them get used up and cannot be used after that. So they are disposed of in such a condition. Secondary cells are known to be rechargeable and thus can be used many times.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




