
Cyclopropane is a ring- chain isomer of:
A. Propane
B. Propene
C. Propyne
D. None of these
Answer
595.2k+ views
Hint: Let's first see the meaning of isomerism, actually it is a phenomenon in which more than one compound is found to have the same chemical formula but different structures. Ring chain isomerism is a type of structural isomerism.
Step by step solution:
- We can see here that there are two types of isomerism that are structural and ring
- Structural isomerism is basically a type of isomerism in which the atoms and the functional groups in the molecule are found to be linked indifferent ways.
In ring – chain isomerism, which is a type of structural isomerism, it is seen that one isomer is having a ring like structure and the other is having an open-chain structure.
- For an example propene and cyclopropane is having the same chemical formula of ${{C}_{3}}{{H}_{6}}$, but they just differ in the way of drawing structures, and it is also found that one of the isomer is having ring like structure and the other is having chain like structure:
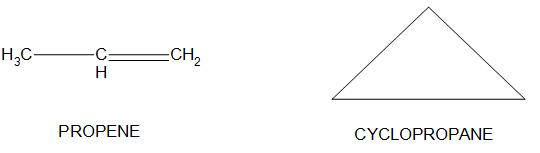
Hence, we can conclude that the correct option is (b) that is Cyclopropane is a ring- chain isomer of propene.
Additional information:
- There are also some of the other types of isomerism found under the category of structural isomerism that are: Chain isomerism, positional isomerism, functional isomerism etc.
- The other type of isomerism is stereoisomerism, which again has two subtypes of isomerism that are: geometric and optical isomerism.
Note:
- It is important to note here that in ring chain isomerism the isomers contain different numbers of pi bonds. In cyclopropane there are 9 sigma bonds and in propene there are 8 sigma bonds and 1pi bond present.
Step by step solution:
- We can see here that there are two types of isomerism that are structural and ring
- Structural isomerism is basically a type of isomerism in which the atoms and the functional groups in the molecule are found to be linked indifferent ways.
In ring – chain isomerism, which is a type of structural isomerism, it is seen that one isomer is having a ring like structure and the other is having an open-chain structure.
- For an example propene and cyclopropane is having the same chemical formula of ${{C}_{3}}{{H}_{6}}$, but they just differ in the way of drawing structures, and it is also found that one of the isomer is having ring like structure and the other is having chain like structure:
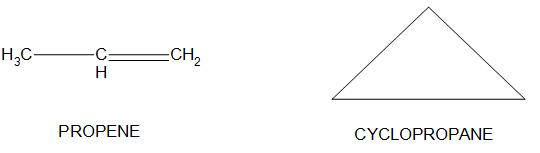
Hence, we can conclude that the correct option is (b) that is Cyclopropane is a ring- chain isomer of propene.
Additional information:
- There are also some of the other types of isomerism found under the category of structural isomerism that are: Chain isomerism, positional isomerism, functional isomerism etc.
- The other type of isomerism is stereoisomerism, which again has two subtypes of isomerism that are: geometric and optical isomerism.
Note:
- It is important to note here that in ring chain isomerism the isomers contain different numbers of pi bonds. In cyclopropane there are 9 sigma bonds and in propene there are 8 sigma bonds and 1pi bond present.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




