
Cyclopentadiene is much more acidic than cyclopentane. The reason is that:
(a)Cyclopentadiene has conjugated double bonds
(b)Cyclopendadiene has both and hybridised carbon atoms
(c)Cyclopentadiene is a strain-free cyclic system
(d)Cyclopentadiene ion, the conjugate base of cyclopentadiene, is an aromatic species and hence has higher stability.
Answer
591.9k+ views
Hint: The property of cycloalkenes which increases the stability of a molecule due to the delocalisation of pi orbitals is called aromaticity. For a molecule to be aromatic, it should be cyclic, have alternating double bonds in resonance, have 4n+2 electrons(where n is a positive integer) and should be planar. Cyclopentadienyl ions have alternate double bonds which can undergo resonance and are aromatic too.
Complete answer:
-When a molecule is said to be aromatic, then it would be conjugated cycloalkene with increased stability due to the delocalization of electrons present in its pi orbital. Aromatic molecules are very stable and cannot break easily.
-For a molecule to be aromatic, there are a set of rules. These rules are
-The molecule should be cyclic and planar.
-Should obey Huckel’s Rule, which states that all the aromatic compounds should have 4n+2 number of pi electrons, where n is a positive integer like 1, 2, 3......
-Every atom of the ring in the structure should have a p-orbital ring which is in perpendicular form to the ring and makes the molecule planar.
Now let us consider cyclopentadiene. The structure of cyclopentadiene is shown in the figure below,
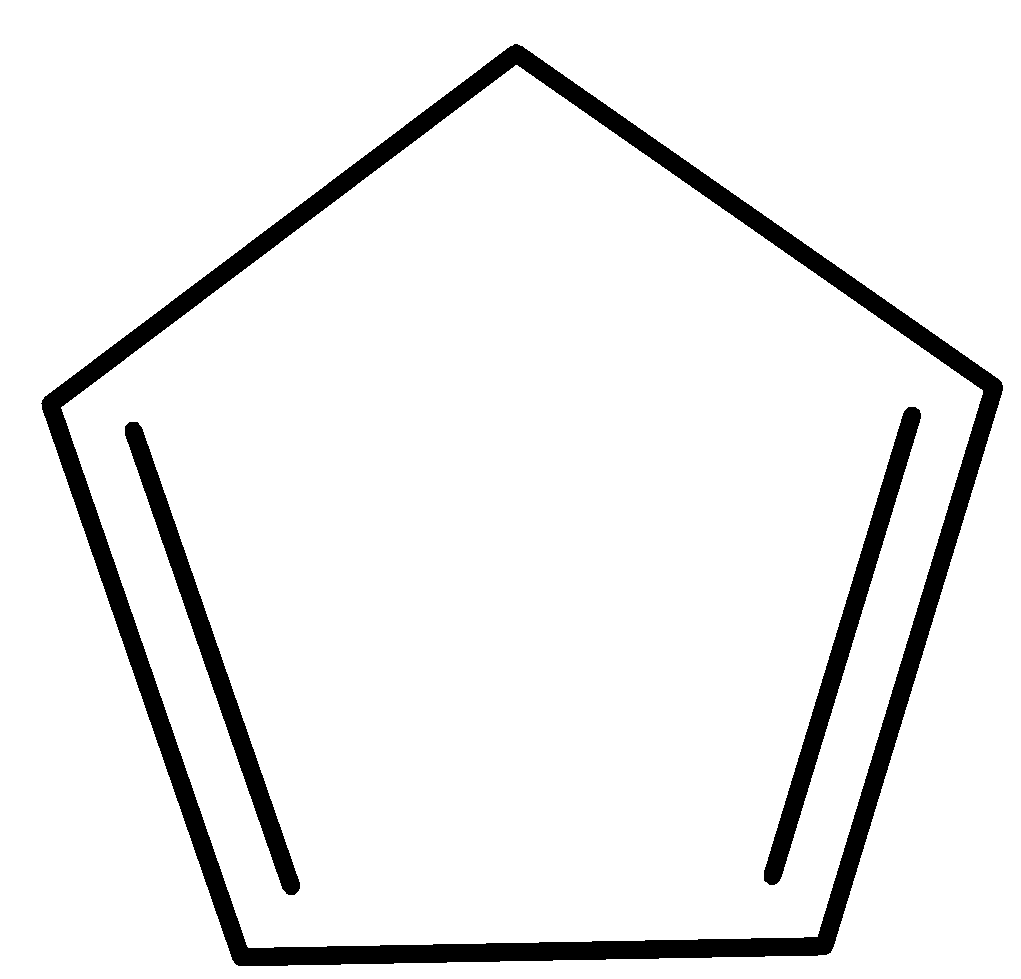
When it loses a proton, its structure becomes
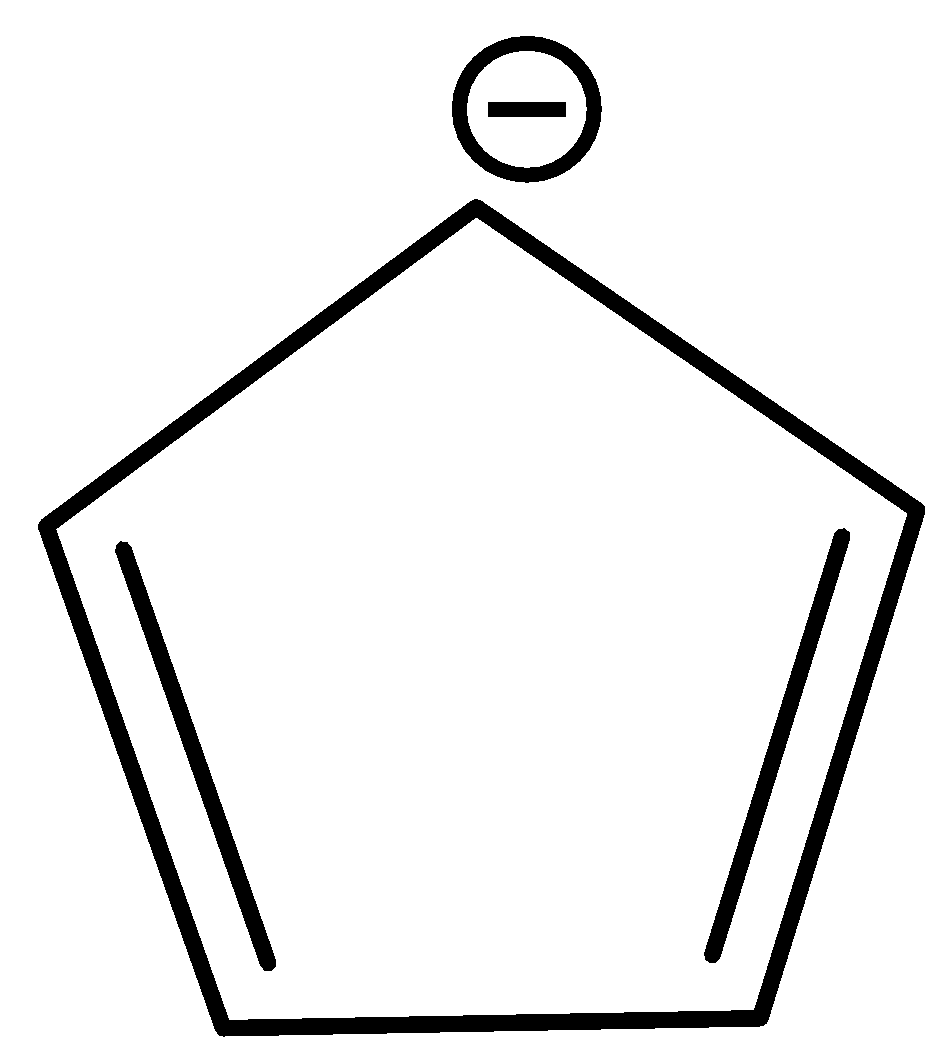
The above structure obeys all the rules of aromaticity. It is cyclic and its planar and has p-orbitals on each atom.
To check for Huckel’s rule, we should count the number of pi electrons in the above molecule. There are 2 pi bonds. Each pi bond contributes 2 electrons. So, 4 electrons. There is a carbanion contributing 2 electrons. So, in total there are 4 electrons. Thus,
4n+2=6
n=1
Therefore, Cyclopentadienyl anion is aromatic and highly stable. So, the tendency of cyclopentadiene to form its anion by losing its proton (from its fifth carbon atom) to get stabilized, is more.
In case of cyclopentane, there is no double bond in it. By losing one proton from its fifth carbon atom leads to a cyclopentane anion which is unstable. So, its tendency to lose proon decreases.
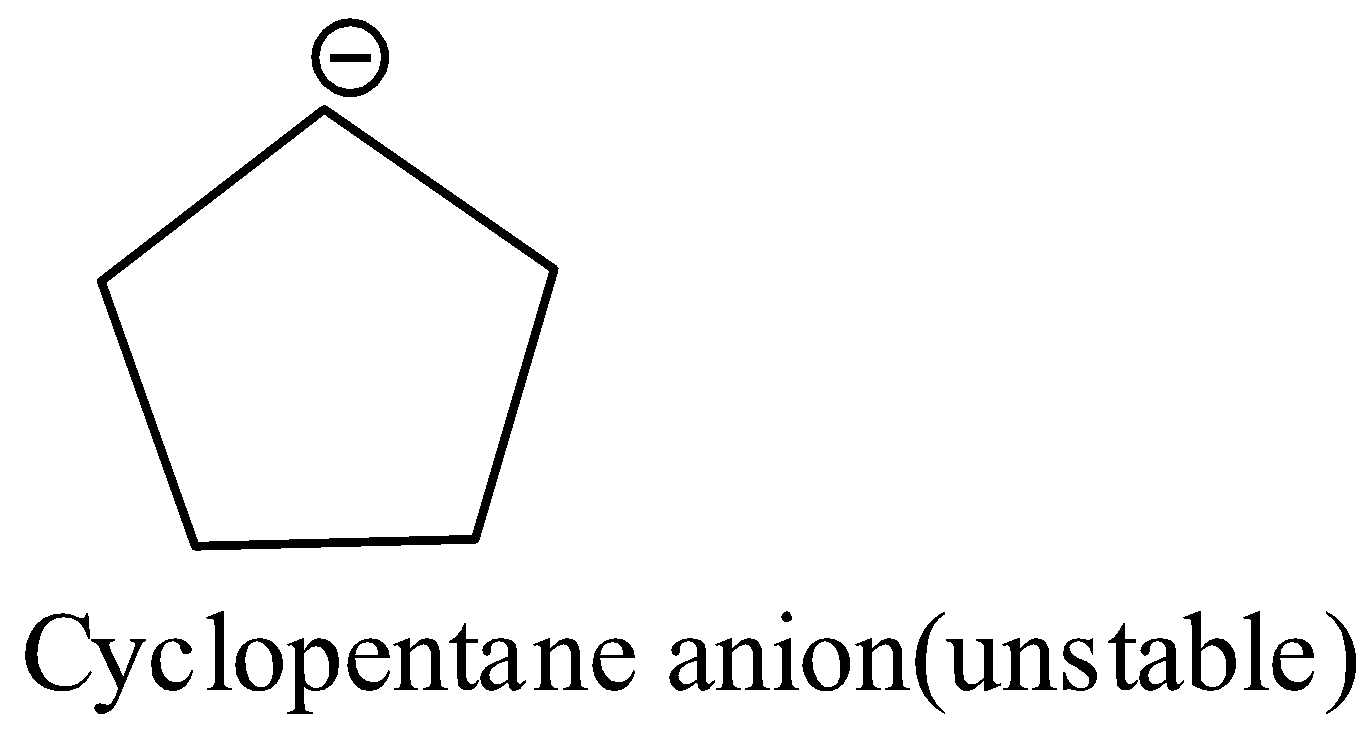
Tendency to lose a proton gives the acidic nature of a molecule. Therefore, cyclopentadiene is acidic due to the presence of conjugated double bonds and it is acidic than cyclopentane.
The correct to the question is option (d).
Note:
Option (a) is also correct to an extent. Cyclopentadiene has conjugate bonds which are in resonance and contribute to the stability of the carbanion formed. But aromaticity provides extra stability. That is why option (d) is correct.
Complete answer:
-When a molecule is said to be aromatic, then it would be conjugated cycloalkene with increased stability due to the delocalization of electrons present in its pi orbital. Aromatic molecules are very stable and cannot break easily.
-For a molecule to be aromatic, there are a set of rules. These rules are
-The molecule should be cyclic and planar.
-Should obey Huckel’s Rule, which states that all the aromatic compounds should have 4n+2 number of pi electrons, where n is a positive integer like 1, 2, 3......
-Every atom of the ring in the structure should have a p-orbital ring which is in perpendicular form to the ring and makes the molecule planar.
Now let us consider cyclopentadiene. The structure of cyclopentadiene is shown in the figure below,

When it loses a proton, its structure becomes

The above structure obeys all the rules of aromaticity. It is cyclic and its planar and has p-orbitals on each atom.
To check for Huckel’s rule, we should count the number of pi electrons in the above molecule. There are 2 pi bonds. Each pi bond contributes 2 electrons. So, 4 electrons. There is a carbanion contributing 2 electrons. So, in total there are 4 electrons. Thus,
4n+2=6
n=1
Therefore, Cyclopentadienyl anion is aromatic and highly stable. So, the tendency of cyclopentadiene to form its anion by losing its proton (from its fifth carbon atom) to get stabilized, is more.
In case of cyclopentane, there is no double bond in it. By losing one proton from its fifth carbon atom leads to a cyclopentane anion which is unstable. So, its tendency to lose proon decreases.


Tendency to lose a proton gives the acidic nature of a molecule. Therefore, cyclopentadiene is acidic due to the presence of conjugated double bonds and it is acidic than cyclopentane.
The correct to the question is option (d).
Note:
Option (a) is also correct to an extent. Cyclopentadiene has conjugate bonds which are in resonance and contribute to the stability of the carbanion formed. But aromaticity provides extra stability. That is why option (d) is correct.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




