
Correct order of $p{K_a}$ is:
(i)
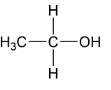
(ii)
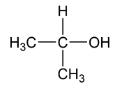
(iii)
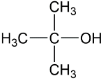
A.$(i) > \,(ii) > \,(iii)$
B.$(ii) > \,(i) > \,(iii)$
C.$(iii) > \,(ii) > \,(i)$
D.$(ii) > \,(iii) > \,(i)$



Answer
547.5k+ views
Hint:$p{K_a}$ value is inversely proportional to the acidic strength but directly proportional to basic strength. So we find out the order of $p{K_a}$ value if we know the acidic strength order. Reversing the order of the acidic strength we can easily write the $p{K_a}$ order. Thus we can write as $p{K_a}\,\alpha \,\,\dfrac{1}{{Acidic\,strength}}$ and also $p{K_a}\,\alpha \,\,Basic\,strength$ .
Complete step-by-step answer:For finding the acidic strength order, we have to first make the conjugate base by removing a proton from acid and then compare the stability of conjugate base. If the conjugate base is stable it means it acts as a weak base thus the acid from it is made up of is strong in nature. Let’s take an example of option (i) which is ethyl alcohol.

Here, the conjugate base of ethyl alcohol is having one $( - C{H_3})$ group which shows $( + I)\,effect$ thus the conjugate base is less stable hence ethyl alcohol is strong in nature. The comparison will show the effect of all other options.
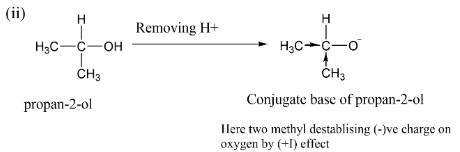
If we see option (ii), here we have $propan - 2 - ol$ after making its conjugate base we see that there are two methyl groups $( - C{H_3})$ which shows $( + I)\,effect$ and thus due to which the $( - )ve\,$ charge is more unstable than ethyl alcohol.
In the last option we have isopropyl alcohol which is having three methyl groups $( - C{H_3})$ having $( + I)\,effect$ by which electron density increases more on oxygen atom thus here also negative charge is not so stable.
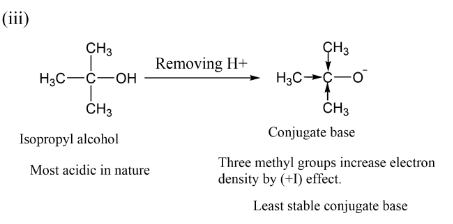
Therefore, we can say that conjugate base is least stable. Among the three alcohols we can say the conjugate base is very stable in option (i) and thus ethyl alcohol is least acidic. While in isopropyl alcohol, the conjugate base is least stable and that is strongly acidic in nature.
$Order\,of\,acidic\,strength:\,(iii) > \,(ii) > \,(i)$
Thus the order of $p{K_a}$ will be reverse of it by this formula $p{K_a}\,\alpha \,\,\dfrac{1}{{Acidic\,strength}}$
$Order\,of\,p{K_a}:\,(i) > \,(ii) > \,(iii)$ Final answer.
Note:In case of amines we usually find out the basic strength, which is the ability to donate lone pairs of electrons on nitrogen. The amine which donates lone pair easily will be strong in nature and the amine which does not donate will have least basic strength. We can find the acidic strength on making the conjugate base and then checking the stability of the conjugate base.
Complete step-by-step answer:For finding the acidic strength order, we have to first make the conjugate base by removing a proton from acid and then compare the stability of conjugate base. If the conjugate base is stable it means it acts as a weak base thus the acid from it is made up of is strong in nature. Let’s take an example of option (i) which is ethyl alcohol.

Here, the conjugate base of ethyl alcohol is having one $( - C{H_3})$ group which shows $( + I)\,effect$ thus the conjugate base is less stable hence ethyl alcohol is strong in nature. The comparison will show the effect of all other options.
If we see option (ii), here we have $propan - 2 - ol$ after making its conjugate base we see that there are two methyl groups $( - C{H_3})$ which shows $( + I)\,effect$ and thus due to which the $( - )ve\,$ charge is more unstable than ethyl alcohol.

In the last option we have isopropyl alcohol which is having three methyl groups $( - C{H_3})$ having $( + I)\,effect$ by which electron density increases more on oxygen atom thus here also negative charge is not so stable.

Therefore, we can say that conjugate base is least stable. Among the three alcohols we can say the conjugate base is very stable in option (i) and thus ethyl alcohol is least acidic. While in isopropyl alcohol, the conjugate base is least stable and that is strongly acidic in nature.
$Order\,of\,acidic\,strength:\,(iii) > \,(ii) > \,(i)$
Thus the order of $p{K_a}$ will be reverse of it by this formula $p{K_a}\,\alpha \,\,\dfrac{1}{{Acidic\,strength}}$
$Order\,of\,p{K_a}:\,(i) > \,(ii) > \,(iii)$ Final answer.
Note:In case of amines we usually find out the basic strength, which is the ability to donate lone pairs of electrons on nitrogen. The amine which donates lone pair easily will be strong in nature and the amine which does not donate will have least basic strength. We can find the acidic strength on making the conjugate base and then checking the stability of the conjugate base.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




