
What is the correct order of a dipole moment?
(a). ${ CH }_{ 4 } < { NF }_{ 3 } < { NH }_{ 3 } < { H }_{ 2 }O$
(b). ${ NF }_{ 3 } < { CH }_{ 4 } < { NH }_{ 3 } < { H }_{ 2 }O$
(c). ${ NH }_{ 3 } < { NF }_{ 3 } < { CH }_{ 4 } < { H }_{ 2 }O$
(d). ${ H }_{ 2 }O < { NH }_{ 3 } < { NF }_{ 3 } < { CH }_{ 4 }$
Answer
525.8k+ views
- Hint: The dipole moment depends on the structures of the molecules as well as the electronegativity of the atoms present in the molecule. In a molecule, if the dipole moment is in the opposite direction, they cancel out each other and the net dipole moment becomes zero.
Complete step-by-step solution -
The bond dipole moment of a chemical bond is used to measure the polarity of the bond whenever there is charge separation. The charge separation occurs when one of the atoms in the chemical bond is more electronegative than the other. Electronegativity is the property of an atom by the virtue of which it attracts the shared pair of electrons toward itself and thereby creating a bond polarity.
The dipole moment is shown by an arrow and it is a vector quantity that is parallel to the bond axis and in chemistry, it usually points from the negative charge to the positive. The electronegative atom will have the partial negative charge formed and the other atom will have a partial positive charge.
Let us see the dipole moment present in the molecules given in the options and find out the correct order:
> ${ CH }_{ 4 }$ : Methane has a tetrahedral structure. Here the carbon atom is more electronegative. So, the direction of the dipole moment will be from hydrogen atoms towards carbon atoms. Due to its structure, the dipole moments will cancel out each other. That’s why it will have the least dipole moment.
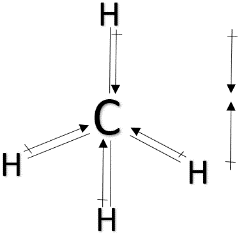
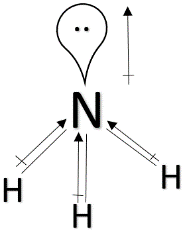
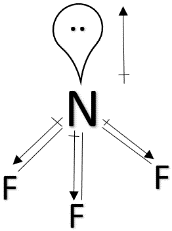
> ${ NF }_{ 3 }$ & ${ NH }_{ 3} $ : Both ${ NF }_{ 3 }$ and ${ NF }_{ 3 }$ have pyramidal structure and a lone pair above nitrogen. But in the case of ${ NH }_{ 3 }$, nitrogen is more electronegative than hydrogen and the dipole moment will be in the direction towards nitrogen. The dipole moment due to the lone pair will also be in the same direction. So, the overall dipole moment will increase. But in the case of ${ NF }_{ 3 }$, fluorine is more electronegative than nitrogen and as a result, the direction of dipole moment will be in the opposite direction than the dipole moment due to the lone pair. So, its dipole moment will decrease. So, ${ NF }_{ 3 } < { NH }_{ 3 }$.
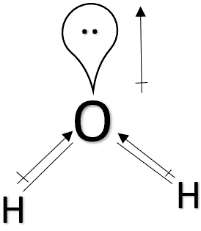
> ${ H }_{ 2 }O$ : Water has a bent shape and a lone pair of electrons above oxygen. Since oxygen is more electronegative than hydrogen, the direction of the dipole moment will be in the direction towards oxygen and the same direction towards the dipole moment of the lone pairs. Since the electronegativity of oxygen is greater than nitrogen, water will have the highest dipole moment among the all.
So, the correct order of dipole moment is ${ CH }_{ 4 } < { NF }_{ 3 } < { NH }_{ 3 } < { H }_{ 2 }O$ which I option A.
Note: For the electrical dipole moment, the direction of the dipole moment is generally considered from the negative charge to the positive charge and in chemistry, it is in the reverse direction. Don’t confuse between them.
Complete step-by-step solution -
The bond dipole moment of a chemical bond is used to measure the polarity of the bond whenever there is charge separation. The charge separation occurs when one of the atoms in the chemical bond is more electronegative than the other. Electronegativity is the property of an atom by the virtue of which it attracts the shared pair of electrons toward itself and thereby creating a bond polarity.
The dipole moment is shown by an arrow and it is a vector quantity that is parallel to the bond axis and in chemistry, it usually points from the negative charge to the positive. The electronegative atom will have the partial negative charge formed and the other atom will have a partial positive charge.
Let us see the dipole moment present in the molecules given in the options and find out the correct order:
> ${ CH }_{ 4 }$ : Methane has a tetrahedral structure. Here the carbon atom is more electronegative. So, the direction of the dipole moment will be from hydrogen atoms towards carbon atoms. Due to its structure, the dipole moments will cancel out each other. That’s why it will have the least dipole moment.

> ${ NF }_{ 3 }$ & ${ NH }_{ 3} $ : Both ${ NF }_{ 3 }$ and ${ NF }_{ 3 }$ have pyramidal structure and a lone pair above nitrogen. But in the case of ${ NH }_{ 3 }$, nitrogen is more electronegative than hydrogen and the dipole moment will be in the direction towards nitrogen. The dipole moment due to the lone pair will also be in the same direction. So, the overall dipole moment will increase. But in the case of ${ NF }_{ 3 }$, fluorine is more electronegative than nitrogen and as a result, the direction of dipole moment will be in the opposite direction than the dipole moment due to the lone pair. So, its dipole moment will decrease. So, ${ NF }_{ 3 } < { NH }_{ 3 }$.


> ${ H }_{ 2 }O$ : Water has a bent shape and a lone pair of electrons above oxygen. Since oxygen is more electronegative than hydrogen, the direction of the dipole moment will be in the direction towards oxygen and the same direction towards the dipole moment of the lone pairs. Since the electronegativity of oxygen is greater than nitrogen, water will have the highest dipole moment among the all.

So, the correct order of dipole moment is ${ CH }_{ 4 } < { NF }_{ 3 } < { NH }_{ 3 } < { H }_{ 2 }O$ which I option A.
Note: For the electrical dipole moment, the direction of the dipole moment is generally considered from the negative charge to the positive charge and in chemistry, it is in the reverse direction. Don’t confuse between them.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




