
What is the coordination number of body-centred cube?
Option
(A) 8
(B) 6
(C) 4
(D) 12
Answer
523.5k+ views
Hint :
The cumulative number of electrons, ions, or molecules bound to an atom in a given molecule or crystal is referred to as the coordination number of that atom. The coordination number of an atom is often referred to as its ligancy. The ligands are the atoms, ions, or molecules that are bonded to the central atom (or molecule/ion). When measuring the coordination number of a central atom in a crystal, the ligancy of molecules is measured differently.
Complete Step By Step Answer:
BCC
A BCC unit cell has an atom in the centre of the structure and atoms at each corner of the cube. A transparent structure is depicted in the diagram below. The atom at the centre of the body, according to this structure, belongs entirely to the unit cell in which it is found.
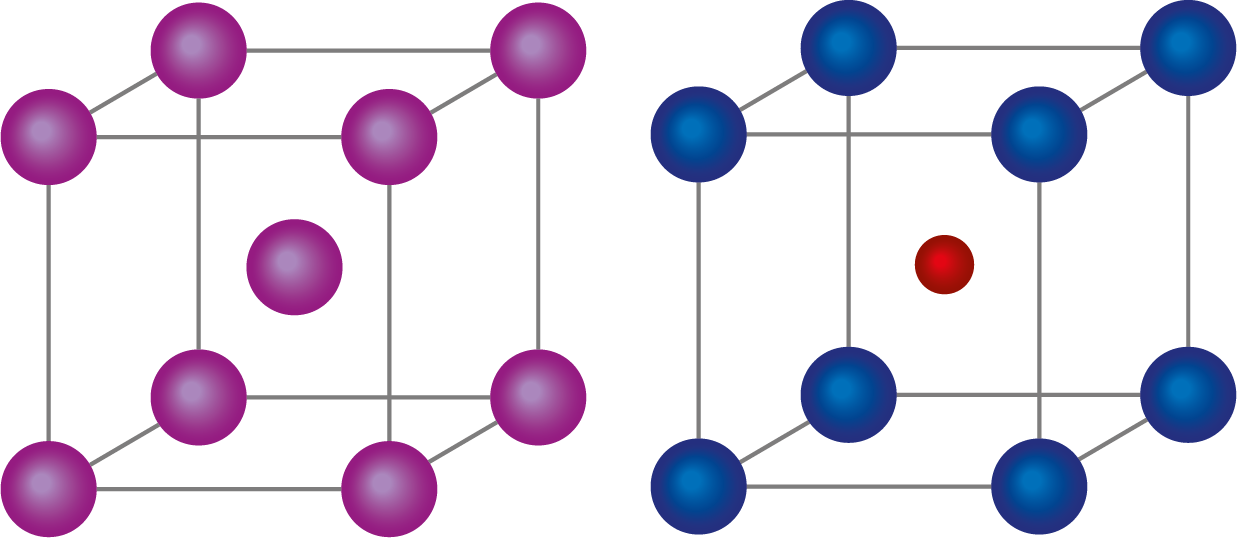
Properties
Any corner of a BCC unit cell has atoms.
At the centre of the structure, there is just one electron.
An transparent structure is depicted in the diagram above.
The atoms at the body's centres, according to this arrangement, belong entirely to the unit cell in which they are found.
The body-centred cube's unit cell contains one atom in each of the eight corners and one atom in the body's middle. As a result, the atom in the centre maintains interaction with the eight atoms in the corners.
As a result, bcc's coordination number is 8.
The cesium ion is in the middle of a cesium chloride crystal, while the chloride ions are in each corner. Both ions have an 8-coordination number.
Note :
The same unit cells are identified in such a way that they occupy the available space without overlapping. A crystal lattice is a three-dimensional structure of electrons, molecules, or ions within a crystal. It consists of a large number of unit cells. Per lattice point is occupied by one of the three constituent particles.
The cumulative number of electrons, ions, or molecules bound to an atom in a given molecule or crystal is referred to as the coordination number of that atom. The coordination number of an atom is often referred to as its ligancy. The ligands are the atoms, ions, or molecules that are bonded to the central atom (or molecule/ion). When measuring the coordination number of a central atom in a crystal, the ligancy of molecules is measured differently.
Complete Step By Step Answer:
BCC
A BCC unit cell has an atom in the centre of the structure and atoms at each corner of the cube. A transparent structure is depicted in the diagram below. The atom at the centre of the body, according to this structure, belongs entirely to the unit cell in which it is found.

Properties
Any corner of a BCC unit cell has atoms.
At the centre of the structure, there is just one electron.
An transparent structure is depicted in the diagram above.
The atoms at the body's centres, according to this arrangement, belong entirely to the unit cell in which they are found.
The body-centred cube's unit cell contains one atom in each of the eight corners and one atom in the body's middle. As a result, the atom in the centre maintains interaction with the eight atoms in the corners.
As a result, bcc's coordination number is 8.
The cesium ion is in the middle of a cesium chloride crystal, while the chloride ions are in each corner. Both ions have an 8-coordination number.
Note :
The same unit cells are identified in such a way that they occupy the available space without overlapping. A crystal lattice is a three-dimensional structure of electrons, molecules, or ions within a crystal. It consists of a large number of unit cells. Per lattice point is occupied by one of the three constituent particles.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




