
How will you convert
i.Benzene to aniline
ii.Benzene to N,N-dimethylaniline
iii.$Cl - {(C{H_2})_4} - Cl$ to ${\text{hexan - 1,6 - diamine}}$
Answer
514.2k+ views
Hint: A chemical reaction in which one functional group on a compound is replaced by another functional group, then this type of reaction is known as substitution reaction. It is of two types i.e., nucleophilic substitution reaction and electrophilic substitution reaction. The conversions given in the question will be performed with the help of substitution reaction.
Complete answer: The given conversions will be achieved as follows:
Benzene to aniline:
Step-1: The benzene reacts with nitric acid in the presence of acidic medium such as sulphuric acid to yield nitrobenzene. It is known as nitration of benzene. The reaction proceeds as follows:
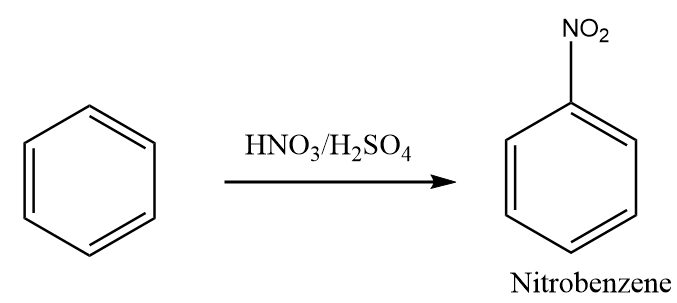
Step-2: The reduction reaction of nitrobenzene in the presence of tin and hydrochloric acid takes place and aniline is formed as the product. The reaction proceeds as follows:
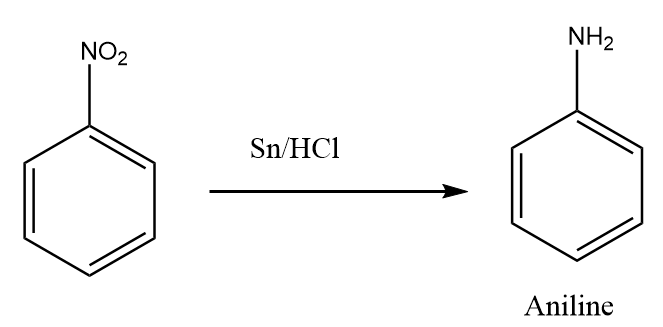
Benzene to N,N-dimethylaniline:
Step-1: The benzene reacts with nitric acid in the presence of acidic medium such as sulphuric acid to yield nitrobenzene. It is known as nitration of benzene. The reaction proceeds as follows:
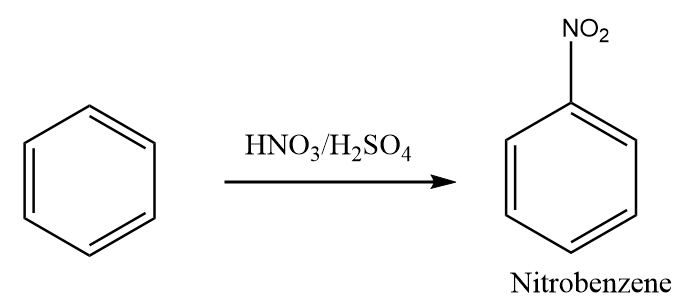
Step-2: The reduction reaction of nitrobenzene in the presence of tin and hydrochloric acid takes place and aniline is formed as the product. The reaction proceeds as follows:
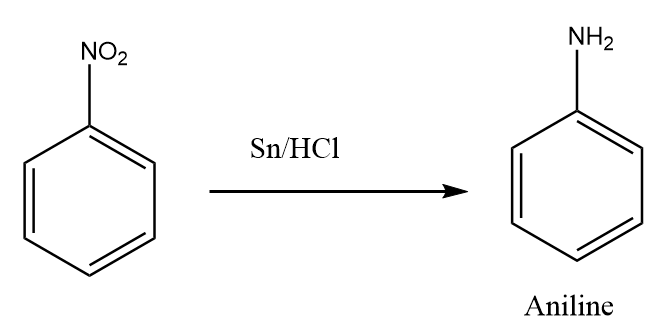
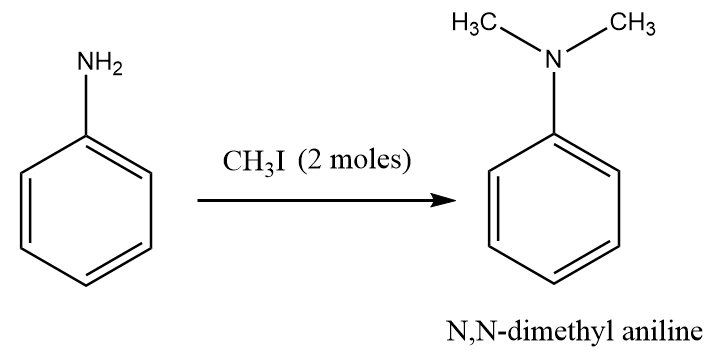
Step-3: Aniline on reaction with two moles of methyl iodide gives N,N-dimethylaniline as the major product. The reaction proceeds as follows:
$Cl - {(C{H_2})_4} - Cl$ to ${\text{hexan - 1,6 - diamine}}$:
Step-1: When the given compound reacts with potassium cyanide in the presence of alcohol, then the chlorine groups are replaced by cyanide groups along with the removal of potassium chloride. The reaction proceeds as follows:
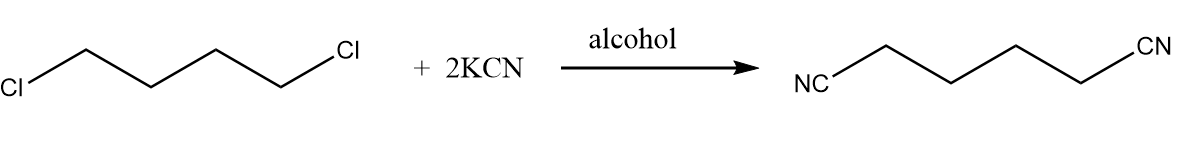
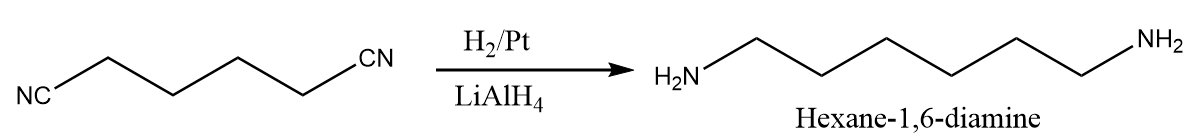
Step-2: Catalytic reduction of cyanide group takes place in the presence of nickel and lithium aluminium hydride and ${\text{hexan - 1,6 - diamine}}$ is obtained as the major product. The reaction proceeds as follows:
Note:
It is important to note that in the reaction of aniline with methyl iodide, the number of moles of methyl iodide must not more than two moles because on increasing the number of moles of methyl iodide, the aniline will have a tendency to undergo Hofmann exhaustive methylation and then desired product will not obtained.
Complete answer: The given conversions will be achieved as follows:
Benzene to aniline:
Step-1: The benzene reacts with nitric acid in the presence of acidic medium such as sulphuric acid to yield nitrobenzene. It is known as nitration of benzene. The reaction proceeds as follows:

Step-2: The reduction reaction of nitrobenzene in the presence of tin and hydrochloric acid takes place and aniline is formed as the product. The reaction proceeds as follows:

Benzene to N,N-dimethylaniline:
Step-1: The benzene reacts with nitric acid in the presence of acidic medium such as sulphuric acid to yield nitrobenzene. It is known as nitration of benzene. The reaction proceeds as follows:

Step-2: The reduction reaction of nitrobenzene in the presence of tin and hydrochloric acid takes place and aniline is formed as the product. The reaction proceeds as follows:

Step-3: Aniline on reaction with two moles of methyl iodide gives N,N-dimethylaniline as the major product. The reaction proceeds as follows:

$Cl - {(C{H_2})_4} - Cl$ to ${\text{hexan - 1,6 - diamine}}$:
Step-1: When the given compound reacts with potassium cyanide in the presence of alcohol, then the chlorine groups are replaced by cyanide groups along with the removal of potassium chloride. The reaction proceeds as follows:
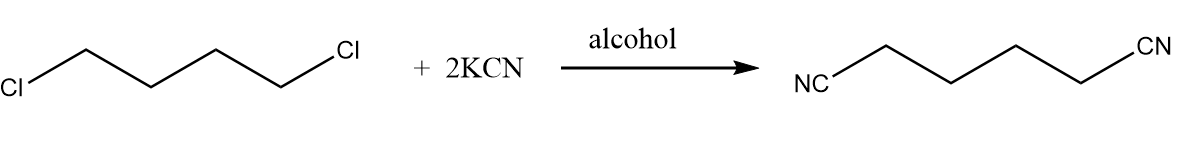
Step-2: Catalytic reduction of cyanide group takes place in the presence of nickel and lithium aluminium hydride and ${\text{hexan - 1,6 - diamine}}$ is obtained as the major product. The reaction proceeds as follows:

Note:
It is important to note that in the reaction of aniline with methyl iodide, the number of moles of methyl iodide must not more than two moles because on increasing the number of moles of methyl iodide, the aniline will have a tendency to undergo Hofmann exhaustive methylation and then desired product will not obtained.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




