
Consider the reactions: Identify A, X, Y and Z.
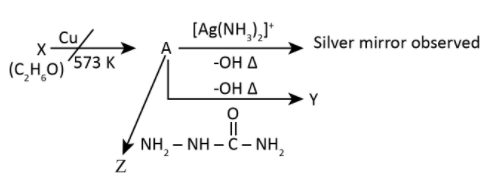
A. A-Ethanol, X-Acetaldehyde, Y-Butanone, Z-Hydrazone
B. A-methoxymethane, X- Ethanoic acid, Y-Acetate ion, Z-Hydrazine
C. A-methoxymethane, X- Ethanol, Y-Ethanoic acid, Z-Semicarbazide
D. A-Ethanal, X-Ethanol, Y- \[but - 2 - {\rm{ }}enal\], Z-Semicarbazone

Answer
574.8k+ views
Hint: In the above conversion, the molecular formula is given which will give us the idea that the molecule with formula \[{C_2}{H_6}O\] is having hydroxyl group. Further, the reagents used can give the idea that molecule A can be a carbonyl group.
Complete answer:
Here from the given question as we know that the silver mirror test is given by the aldehyde and not by ketone hence, the product A in the answer has to be aldehyde. We can eliminate options A, B and C and check the option D for X, Y and Z.
X has to be ethanol because \[{C_2}{H_4}{O_2}\] is the molecular formula for ethanoic acid and the formula for acetaldehyde is \[{C_2}{H_4}O\] which does not matches with the question which ask the reaction for \[{C_2}{H_6}O\].
Hence, X will be alcohol known as ethanol with structural formula as shown in the figure given below.

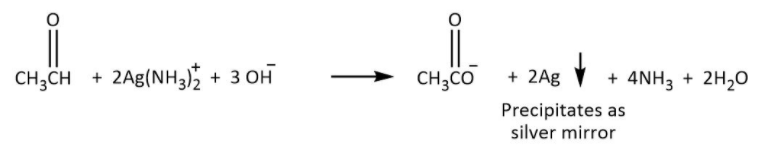
Tollen’s test: It is the test used to differentiate between the ketone and aldehydes. The use of reagent known as ammoniacal silver nitrate is used to detect the Aldehydes. The use of this reagent is for oxidizing aldehyde to form carboxylic acid and the reagent will be reduced to silver. As the aldehyde produced free silver due to reduction of ammoniacal silver nitrate the reaction is also known as Silver mirror test for aldehyde. The number of carbons will remain the same during the reaction.
Hence, the structure of product A which is ethanal will be as shown in the figure above, then it is possible that it will give a silver mirror test. Given by the following reaction in which aldehyde is converted to carboxylic acid.
Reaction of ethanal in the presence of hydroxyl ion will give us \[but - 2 - {\rm{ }}enal\] because that reaction is nothing but aldol condensation. In aldol condensation the two molecules of the aldehyde will combine in presence of base to produce \[\beta - hydroxy \,aldehyde\]which is known as aldol hence, the reaction is aldol condensation reaction. Here as we can see the molecule is ethanal or acetaldehyde which will condense in presence of base like sodium hydroxide to produce \[3 - hydroxybutanal\] it will further dehydrate in presence of acid to give \[but - 2 - {\rm{ }}enal\].
Product Z is \[2 - ethylidene\;hydrazinecarboxamide\] or semicarbazone which is nothing but\[\alpha - \beta \;unsaturated\;aldehyde\] formed due to conversion of aldehyde to semicarbazone derivative in presence of semicarbazide because it contain nitrogen as nucleophile.
Hence, the correct answer is (D) A-Ethanal, X-Ethanol, Y-But-2-enal, Z-Semicarbazone
Note:
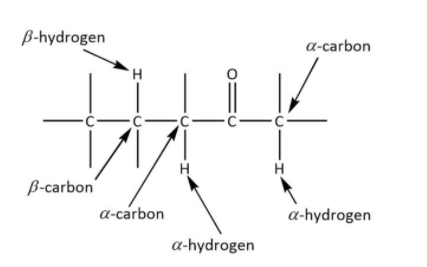
The recognition of alpha and beta carbon is important to decide the product. The given figure shows the correct alpha and beta carbon.
Complete answer:
Here from the given question as we know that the silver mirror test is given by the aldehyde and not by ketone hence, the product A in the answer has to be aldehyde. We can eliminate options A, B and C and check the option D for X, Y and Z.
X has to be ethanol because \[{C_2}{H_4}{O_2}\] is the molecular formula for ethanoic acid and the formula for acetaldehyde is \[{C_2}{H_4}O\] which does not matches with the question which ask the reaction for \[{C_2}{H_6}O\].
Hence, X will be alcohol known as ethanol with structural formula as shown in the figure given below.

Tollen’s test: It is the test used to differentiate between the ketone and aldehydes. The use of reagent known as ammoniacal silver nitrate is used to detect the Aldehydes. The use of this reagent is for oxidizing aldehyde to form carboxylic acid and the reagent will be reduced to silver. As the aldehyde produced free silver due to reduction of ammoniacal silver nitrate the reaction is also known as Silver mirror test for aldehyde. The number of carbons will remain the same during the reaction.
Hence, the structure of product A which is ethanal will be as shown in the figure above, then it is possible that it will give a silver mirror test. Given by the following reaction in which aldehyde is converted to carboxylic acid.

Reaction of ethanal in the presence of hydroxyl ion will give us \[but - 2 - {\rm{ }}enal\] because that reaction is nothing but aldol condensation. In aldol condensation the two molecules of the aldehyde will combine in presence of base to produce \[\beta - hydroxy \,aldehyde\]which is known as aldol hence, the reaction is aldol condensation reaction. Here as we can see the molecule is ethanal or acetaldehyde which will condense in presence of base like sodium hydroxide to produce \[3 - hydroxybutanal\] it will further dehydrate in presence of acid to give \[but - 2 - {\rm{ }}enal\].
Product Z is \[2 - ethylidene\;hydrazinecarboxamide\] or semicarbazone which is nothing but\[\alpha - \beta \;unsaturated\;aldehyde\] formed due to conversion of aldehyde to semicarbazone derivative in presence of semicarbazide because it contain nitrogen as nucleophile.
Hence, the correct answer is (D) A-Ethanal, X-Ethanol, Y-But-2-enal, Z-Semicarbazone
Note:
The recognition of alpha and beta carbon is important to decide the product. The given figure shows the correct alpha and beta carbon.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




