
Consider the following reaction:
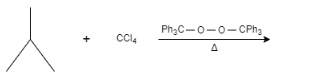
A.
B.
C.
D. No reaction



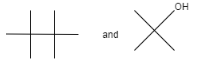
Answer
546.6k+ views
Hint: $2-$ methylpropane is also known as isobutane, which has a molecular formula $HC{{(C{{H}_{3}})}_{3}}$ . It is a colorless and odourless gas, which is an isomer of butane. It is a precursor molecule in the petrochemical industry. It is used as a diluent for making high density polyethylene.
Complete step-by-step answer:
Carbon tetrachloride is an organic compound which has the molecular formula, $CC{{l}_{4}}$ . It is non – flammable at low temperature and therefore it is widely used in fire extinguishers. It is a colorless liquid which is used as a cleaning agent. When exposed to high concentration, it can affect the central nervous system and degenerate the liver.
In this question, when $2-$ methylpropane is reacted with carbon tetrachloride in presence of a radical initiator, it results in the formation of $2-$ chloro $-2-$ methylpropane and chloroform. Let us see the mechanism of this reaction.
1. Step 1:
In the first step, chain initiation step takes place, in which the molecule creates a radical species by homolytic cleavage.
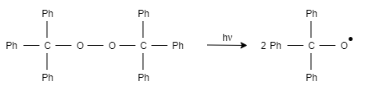
2. Step 2:
This radical species reacts with $2-$ methylpropane and results in the formation of an alcohol and tert-butyl radical.
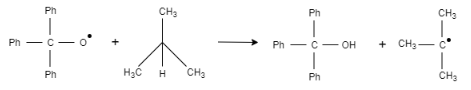
3. Step 3:
This tert-butyl radical is reacted with carbon tetrachloride, which results in the formation of $2-$ chloro $-2-$ methylpropane and trichloromethyl radical.
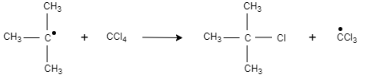
4. Step 4:
Trichloromethyl radical is reacted with $2-$ methylpropane, which results in the formation of $2-$ chloro $-2-$ methylpropane and chloroform.
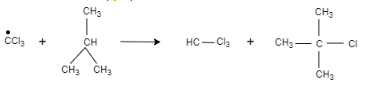
Therefore, the correct option is (A).
Note: It is to be noted that radical initiators are those substances that produce radical species at very mild condition. It also promotes radical reactions. The bonds between these substances are very weak and therefore, they have small bond dissociation energies. $2-$ chloro $-2-$ methylpropane is an organochlorine which is a colorless and flammable liquid.
Complete step-by-step answer:
Carbon tetrachloride is an organic compound which has the molecular formula, $CC{{l}_{4}}$ . It is non – flammable at low temperature and therefore it is widely used in fire extinguishers. It is a colorless liquid which is used as a cleaning agent. When exposed to high concentration, it can affect the central nervous system and degenerate the liver.
In this question, when $2-$ methylpropane is reacted with carbon tetrachloride in presence of a radical initiator, it results in the formation of $2-$ chloro $-2-$ methylpropane and chloroform. Let us see the mechanism of this reaction.
1. Step 1:
In the first step, chain initiation step takes place, in which the molecule creates a radical species by homolytic cleavage.

2. Step 2:
This radical species reacts with $2-$ methylpropane and results in the formation of an alcohol and tert-butyl radical.

3. Step 3:
This tert-butyl radical is reacted with carbon tetrachloride, which results in the formation of $2-$ chloro $-2-$ methylpropane and trichloromethyl radical.

4. Step 4:
Trichloromethyl radical is reacted with $2-$ methylpropane, which results in the formation of $2-$ chloro $-2-$ methylpropane and chloroform.

Therefore, the correct option is (A).
Note: It is to be noted that radical initiators are those substances that produce radical species at very mild condition. It also promotes radical reactions. The bonds between these substances are very weak and therefore, they have small bond dissociation energies. $2-$ chloro $-2-$ methylpropane is an organochlorine which is a colorless and flammable liquid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




