
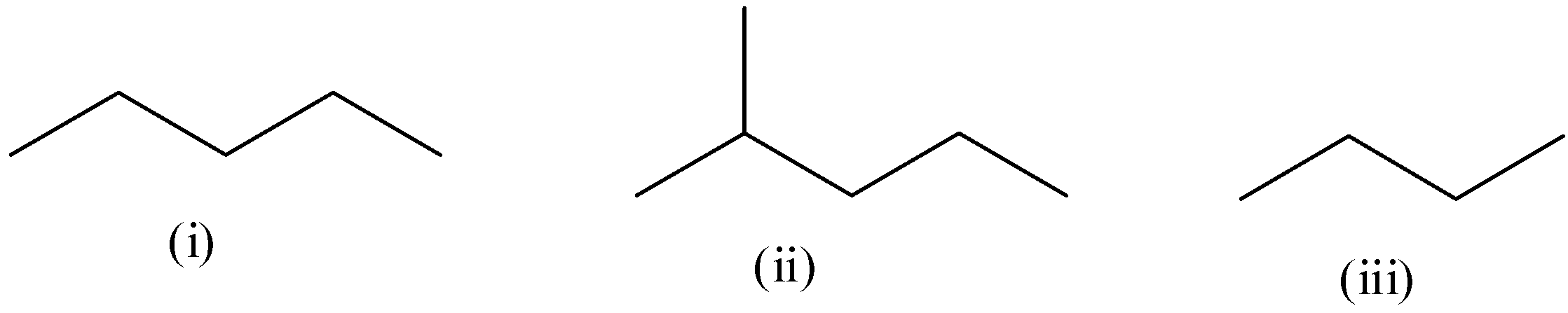
Compare the heats of combustion of the following compounds:

Answer
526.8k+ views
Hint: We know that enthalpy is otherwise known as heat of combustion. We know that in chemical reaction, new bonds are formed and old bonds are broken. For breaking of old bonds, some energy is needed. There must be some quantity of energy present in molecules of reactant to state reaction. In the combustion reaction, the heat generation is because the energy required for old bond breaking is lower when compared to energy needed by new bond formation.
Complete answer:
We need to remember that the exothermic reactions are those reactions where heat is given off when one mole of substance is combusted and the heat is known as heat of combustion. Heat of combustion varies for saturated hydrocarbons and is obtained with rise in the number of atoms of carbons and increase in combustion of heat. This is because of more carbons present for burning and the higher number of bonds which goes through changes.
When oxygen and hydrocarbons are in the form of gases, the combustion reaction gets enabled. A bigger molecule has extra van der Waals attraction which makes it difficult for them to break off from adjacent neighbors and turn them to gas.
When the number of carbon atoms is more, the heat of combustion is also more which means higher number of carbon atoms and heat of combustion are directly proportional to one another.
The total number of atoms of carbon in structure (i) is five.
The total number of atoms of carbon in structure (ii) is six.
The total number of atoms of carbon in structure (iii) is four.
The structure (ii) would have the highest heat of combustion and structure (iii) would have lowest heat of combustion.
Note:
We have to know that primary hydrogen, secondary hydrogen, and tertiary hydrogen are also used to predict the heat of combustion. The carbon which is bonded to primary hydrogen is stronger than carbon which is bonded to secondary hydrogen and carbon which is bonded to tertiary hydrogen. When the number of primary hydrogen is higher, the heat of combustion would be more. Properties like angle strain are considered, when the number of hydrogen is similar.
Complete answer:
We need to remember that the exothermic reactions are those reactions where heat is given off when one mole of substance is combusted and the heat is known as heat of combustion. Heat of combustion varies for saturated hydrocarbons and is obtained with rise in the number of atoms of carbons and increase in combustion of heat. This is because of more carbons present for burning and the higher number of bonds which goes through changes.
When oxygen and hydrocarbons are in the form of gases, the combustion reaction gets enabled. A bigger molecule has extra van der Waals attraction which makes it difficult for them to break off from adjacent neighbors and turn them to gas.
When the number of carbon atoms is more, the heat of combustion is also more which means higher number of carbon atoms and heat of combustion are directly proportional to one another.
The total number of atoms of carbon in structure (i) is five.
The total number of atoms of carbon in structure (ii) is six.
The total number of atoms of carbon in structure (iii) is four.
The structure (ii) would have the highest heat of combustion and structure (iii) would have lowest heat of combustion.
Note:
We have to know that primary hydrogen, secondary hydrogen, and tertiary hydrogen are also used to predict the heat of combustion. The carbon which is bonded to primary hydrogen is stronger than carbon which is bonded to secondary hydrogen and carbon which is bonded to tertiary hydrogen. When the number of primary hydrogen is higher, the heat of combustion would be more. Properties like angle strain are considered, when the number of hydrogen is similar.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




